Fig. 8.
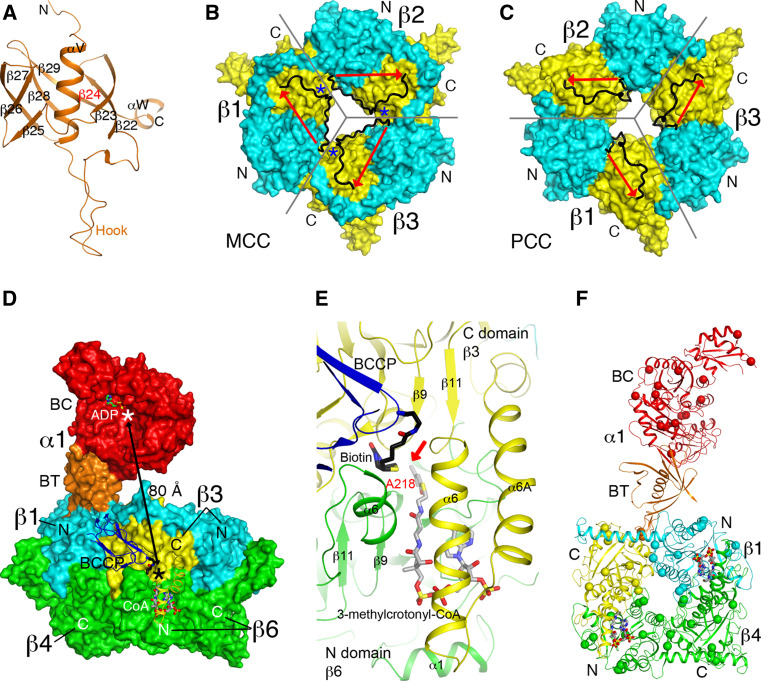
Structure of P. aeruginosa MCC. a Structure of the BT domain of the P. aeruginosa MCC α subunit. The missing third strand is indicated in red. b Structure of the β6 hexamer of MCC. The subunit boundaries are indicated with the gray lines. The N (cyan) and C (yellow) domains are labeled. The linker between the two domains is shown in black, with the direction given by the red arrow. The linkers from neighboring subunits come very close to each other at one point (blue asterisk), and therefore only a small change is needed to switch to the connectivity seen in the PCC β subunit. c Structure of the β6 hexamer of PCC, shown in the same scheme as panel B. The linker in PCC β runs in the opposite direction compared to MCC β. d Relationship between the BC and CT active sites (indicated with the asterisks) in the MCC holoenzyme. While the BT domain of the α subunit contacts one β2 dimer (β1 and β4), the BCCP domain of that α subunit is actually located in the active site of a different β2 dimer (β3 and β6). e Binding modes of biotin (black) and 3-methylcrotonyl-CoA (gray) to the CT active site of MCC. The position of 3-methylcrotonyl-CoA is modeled based on that of CoA in MCC [23] and crotonyl-CoA in GCDα [30]. The carbon atom to be carboxylated is indicated with the red arrow. f Locations of disease-causing missense mutations in the structure of MCC (indicated with the spheres). Only the β1–β4 dimer is shown