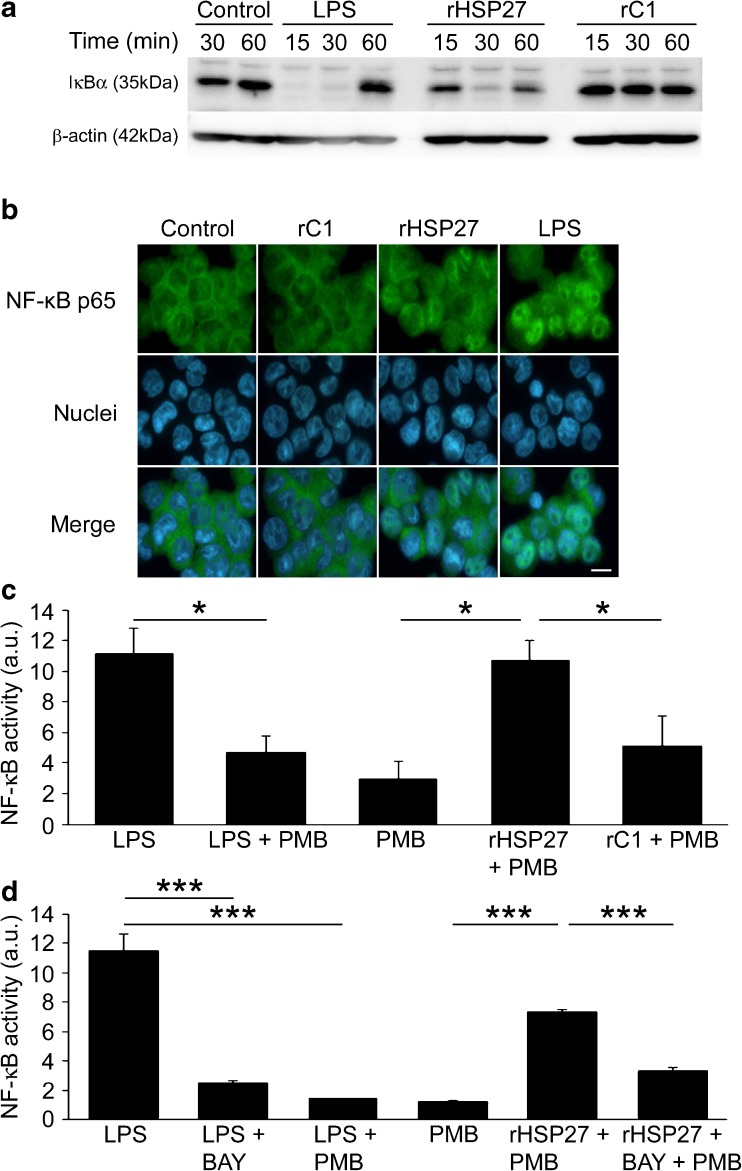
Fig. 1.
a IκBα degradation in rHSP27-treated macrophages. PMA-differentiated THP-1 cells were treated with either LPS (10 ng/mL), rHSP27 (9.6 μM), or rC1 (9.6 μM) for the times indicated. Equal amounts of whole cell lysates were subject to Western blotting with an antibody directed against IκBα. β-Actin was used as a loading control. b Nuclear translocation of the NF-κB p65 subunit in response to rHSP27. PMA-differentiated THP-1 cells were treated with media alone (control), rC1 (9.6 μM), rHSP27 (9.6 μM), or LPS (10 ng/mL) for 30 min. The cells were then stained with an antibody to p65 (green) and the nuclear stain Hoechst (blue). Scale bar = 10 μm. c NF-κB activity in rHSP27-treated macrophages. NF-κB reporter assays were performed using THP-1 Blue macrophages. PMA-differentiated cells were treated with LPS (10 ng/mL), Polymyxin B (10 μg/mL), rHSP27 (9.6 μM), or C1 (9.6 μM) for 24 h. Cell supernatants were then analyzed for presence of secreted embryonic alkaline phosphatase (SEAP) as a reporter of NF-κB activation. One-way ANOVA *p < 0.05; n = 3–4. d Reduction of rHSP27-induced NF-κB activity in the presence of BAY 11-7082, an inhibitor of IκBα phosphorylation. Inhibition of NF-κB signaling was achieved by pre-treating the cells for 1 h with BAY 11-7082 (10 μM). One-way ANOVA ***p < 0.001; n = 3