Abstract
Heat shock protein 60 (hsp60) is a highly conserved stress protein and target of self-reactive T cells in various inflammatory diseases. Not much is known about a possible role in atopic disease. As atopic diseases are considered to be the result of a disturbance in the balance between T helper cells type 2 and regulatory T cells, it is of interest to know whether hsp60 acts as a bystander antigen in atopic disease. Our aim was to investigate whether hsp60 is involved in the chronicity of inflammation of atopic dermatitis (AD). We studied the expression of hsp60 in skin tissue of adults with AD by immunohistochemistry. Peripheral blood mononuclear cells (PBMC) of children with AD were cultured with hsp60 and proliferative responses, cytokine secretion, surface markers, and functional assays were compared to responses of PBMC of healthy controls (HC). Hsp60 was detected more in lesional skin of AD patients compared to nonlesional skin. Furthermore, PBMC of children with AD proliferated more strongly in response to hsp60 compared to HC. hsp60-reactive T cells of atopic children produced high levels of IFNγ and low levels of IL-10. In vitro activation with hsp60 leads to the induction of CD4+CD25bright T cells expressing FOXP3 in both HC as well as in atopic children. However, despite their regulatory phenotype, hsp60-induced CD4+CD25brightCD127−FOXP3+ T cells of AD patients were incapable of suppressing effector T cells in vitro. hsp60 is recognized by proinflammatory (IFNγ high, IL-10 low) T cells in atopic patients and is more present in lesional AD skin. This suggests that hsp60-specific T cell responses contribute to local inflammation in AD.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-012-0361-3) contains supplementary material, which is available to authorized users.
Keywords: Atopy, Atopic dermatitis, Regulatory T cells, Human heat shock protein 60
Introduction
Atopic dermatitis (AD) is a chronic skin condition in patients with atopic disease. Atopic diseases are inflammatory disorders caused by the induction of a TH2 response by an allergen. Increasing evidence demonstrates that allergy is not a mere result of an over-reactive TH2 response, but that immune regulatory cells such as natural killer T cells and regulatory T cells (Tregs) play a role in the regulation of this balance (Akdis and Akdis 2009; Hawrylowicz and O’Garra 2005; Umetsu et al. 2003; Werfel and Wittmann 2008). These cell populations can suppress effector T cell responses, both by cell–cell contact and by the secretion of regulatory cytokines as IL-10 and TGF-β. In active atopic diseases, several studies show that the suppressive function of Tregs is diminished (Bellinghausen et al. 2003; Ling et al. 2004; Tiemessen et al. 2004). Thus, Tregs form an attractive target for therapy in allergic diseases. Studies in various experimental models of autoimmunity have revealed that Tregs can be targeted in both an antigen-specific and an antigen-nonspecific fashion (Roord et al. 2006). Both venues of immune therapy are now currently being explored in various autoimmune diseases.
Specific targeting of Tregs with antigens has the advantage that only a subpopulation of T cells is manipulated which lowers the risk of adverse effects. However, the main dilemma of antigen-specific therapy in humans is to find an antigen which is suitable. In some inflammatory diseases, dominant disease-provoking antigens or allergens are well-known and these can be used for antigen-specific therapy. Indeed, the proof of principle of such an approach was underscored by Alexander et al. who showed that peptides of Fel d 1 (the major cat allergen) can induce antigen-specific tolerance in cat allergic patients (Alexander et al. 2005). In AD, as in many other chronic inflammatory diseases, a single disease-triggering antigen is not known. For such a disease, an antigen-specific approach is still feasible if an antigen can be identified that fulfils at least two conditions. First, the antigen needs to be recognized by T cells from patients and, second, the antigen must be available at the site of inflammation, preferably in a disease-specific fashion. Heat shock proteins (hsp) could fit this profile. Hsp are immunodominant antigens and a common target of T cell recognition in various inflammatory diseases (Albani et al. 2011; de Kleer et al. 2010; Kamphuis et al. 2005; van Eden et al. 2005). Especially hsp60 seems to be an important regulating antigen in human inflammatory diseases, as it is capable of enhancing the regulatory function of human Tregs and thereby dampening the inflammatory process (van Eden et al. 2012; Zanin-Zhorov et al. 2006).
Thus, as hsp60 can promote induction of (adaptive) Tregs, it is conceivable that it could play a role not only in classical TH1 diseases but also TH2 diseases. Indeed, in asthma, a much studied TH2 disease, upregulation of several hsp has been shown not only in macrophages and circulating CD4+ T cells but also in epithelial cells (Guajardo et al. 2005; Kapitein et al. 2008; Madore et al. 2010), but it is unknown what the functional consequences could be. We hypothesized that upregulation and subsequent T cell immune recognition of self-hsp60 could as well take place in a typical TH2 disease such as AD. Therefore, our aim was to investigate whether hsp60 is a target for T cells in AD and thus could play a role in the chronicity of local inflammation.
Methods
Patients and control subjects
Fifty-two atopic children and 30 healthy controls (HC) were included in this study. Eligible patients were children suffering from physician-diagnosed AD as defined by Hanifin and Rajka (Rajka 1989). To assure underlying atopy, all children needed to have a positive radioallergosorbent test for at least one of the three common food allergens, egg, cow’s milk, or peanut, with a history of a clinical allergic reaction after ingestion of the protein (either parental history (Sampson score ≥ 3) or by food challenge). Patient characteristics are given in Table 1. HC were children who underwent a urological or orthopedic surgical procedure. None of them had a history of allergy or a recent infection nor a first-degree relative with a history of allergy or asthma. Both written and oral information about the study was given to the parents and written informed consent from the parents was obtained. The study has been approved by the Medical Ethics Committee of the University Medical Centre, Utrecht, The Netherlands. Patient samples were used for different assays and measurements based on the availability of T cells and serum.
Table 1.
Patient characteristics
| Atopic patients (n = 55) | HC (n = 30) | |
|---|---|---|
| Male (%) | 71 % | 78 % |
| Age, mean (range) | 8.8 (1.5–17.5) | 8.6 (1.2–17.3) |
| Eczema at presentation | 51 (93 %) | 0 |
| Food allergy | 55 (100 %) | 0 |
| Asthma | 24 (44 %) | 0 |
| Skin prick test positive | 47 (87 %) | 0 |
| Elevated IgE | 55 (100 %) | 0 |
| Positive food | 31 (56 %) | 0 |
Immunohistochemistry
Biopsy specimens (3 mm) were taken from four adult volunteers suffering from moderate to severe AD (for ethical reasons, only adult volunteers were used) under local anesthesia (Xylocaine) and snap-frozen in liquid nitrogen. The lesional biopsy was taken from an active lesion, whereas the nonlesional biopsy was taken from healthy-looking skin. Approval was given by the Medical Ethics Committee of the University Medical Centre, Utrecht, The Netherlands.
Subsequently the biopsies were embedded in Tissuetek® (Sakura, Torrance, CA, USA) and stored at −80 °C until further handling. The antibody used as marker for immunohistochemical staining of the frozen sections was antihuman hsp60 (LK2, kind gift from P. van Kooten, Department of Veterinary Medicine, University of Utrecht, The Netherlands) and mouse antihuman CD3 (cat#347340, used 1:50; Becton Dickinson (BD) Biosciences, San Jose, CA, USA). Single staining for hsp60 and CD3 combined with a biotinylated horse antimouse IgG (Vector Laboratories, Inc., Burlingame, CA, USA) was performed as described previously. Stainings were performed on sequential slides to compare hsp60 and CD3 staining in comparable locations. Skin sections were examined by light microscopy.
Proliferation and direct culture assays with PBMC
Peripheral blood mononuclear cells (PBMC) were isolated and cultured as described previously (de Jager et al. 2003). For direct cultures, cells were cultured for 7 days in the absence or presence of 10 μg/ml low endotoxin human hsp60 (0.3 pg/μg protein; Faculty of Veterinary Medicine, University of Utrecht, Utrecht, The Netherlands). Concanavalin A (2.5 μg/ml; Calbiochem, La Jolla, CA USA) and tetanus toxoid (1.5 μg/ml; RIVM, Bilthoven, The Netherlands) were used as positive controls. A mouse class II restricted peptide (Ova) was used as an irrelevant control. For the proliferation assays, cells were cultured for 96 h only. For the final 16 h of culture, 1 μCi/well [3H]thymidine (ICN Biomedicals, Amsterdam, The Netherlands) was added to each well. Cells were harvested according to standard procedures and incorporated radioactivity was measured by a liquid scintillation counter and expressed as counts per minute. The magnitude of the proliferative response is expressed as the stimulation index (SI), which is the mean counts per minute of cells cultured with antigen divided by the mean counts per minute of cells cultured without antigen. As additional control on the effect of possible lipopolysaccharide (LPS) contamination of hsp60, proliferation assays were performed in a control group of patients after treatment of hsp60 (10 μg/ml) or LPS (10 μg/ml; Sigma-Aldrich Corp, St. Louis, MO, USA), with 10 μg/ml polymyxin B (Bio-Rad Laboratories, Hercules CA, USA) for 1 h at room temperature or heat inactivation at 95 °C for 30 min.
Multiplexed particle-based immunoassay
Cytokine levels were measured after the activation of lymphocytes in vitro as described above. After 96 h, the supernatants of the cell cultures were stored at −80 °C until analysis. Cytokines levels of IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, and IFNγ were measured with the Bio-Plex system and analyzed with the Bio-Plex Manager software version 6.0 (Bio-Rad) which uses the Luminex xMap technology (de Jager et al. 2005). The peptide-specific cytokine production is calculated as the cytokine production of cells cultured with peptide subtracted with the cytokine production of cells cultured without peptide.
Lymphocyte cell surface markers
At day 7 from the direct cell culture assays as described above, PBMC were harvested and prepared for fluorescence-activated cell sorting (FACS) staining as described previously (de Kleer et al. 2003). The cells were incubated in 50 μl FACS buffer containing three appropriately diluted phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, or Cy-Chrome-labeled monoclonal antibodies against human CD4 (clone RPA-T4), CD25 (clones: activated protein C [APC], M-A251; PE, 2A3), CD30 (clone: Ber-H83), and CD69 (clone: FN50). Stained mononuclear cells were diluted in FACS fluid and run on a FACSCalibur (BD Biosciences). CellQuest software (BD Biosciences) was used for analysis.
Cytokine analysis by lymphocyte intracellular staining and flow cytometry
Direct T cell lines were generated as described above. During the last 4 h of culture, GolgiStop (BD Biosciences) was added (final concentration of 2 μM). The cells were harvested and stained for intracellular cytokine and FOXP3 analysis as described previously. For the staining, a predetermined optimal concentration of PE-conjugated anti-IL-10 (clone JES3-19F1), anti-IL-4 (clone MP4-25D2), FITC-conjugated anti-IFNγ (clone 4S.B3; BD Biosciences), and APC-conjugated FOXP3 (Ebioscience, San Diego, CA, USA) was added to the cells. Cells were analyzed as described above.
Functional assays
To test the induced CD4+CD25+FOXP3+ Tregs on suppressive function, PBMC were cultured for 96 h as described above in the presence of hsp60. CD4+CD25+CD127− T cells (which were used to quantify FOXP3+ T cells in the functional assay) and CD4+CD25− effector T cells from the same donor were directly sorted by FACS (FACS Aria, BD Biosciences) into plate-bound anti-CD3-coated wells (OKT-3, 1 μg/ml) in different ratios (1:0, 1:0.5, 1:1, and 1:2). T cell-depleted PBMC from the same donor were irradiated (3,500 Rad) and were used as antigen-presenting cells (1:10). The cells were incubated at 37 ° C for 96 h, with the last 16 h of incubation in the presence of [3H]thymidine (1 μCi/well). The suppressive activity was determined by calculating the percentage difference in proliferative response (mean [3H]thymidine incorporation (counts per minute) of triplicate wells) between CD4+CD25− T cells cultured alone and CD4+CD25− T cells cultured in the presence of induced CD4+CD25+CD127− T cells.
Statistical analysis
Basic descriptive statistics were used to describe the patient population. A nonparametric test (Mann–Whitney U test, two-sided) was applied to determine significant differences between the patient and control groups regarding proliferative responses, cytokine production, and expression of cell surface markers. Where mentioned, values are noted as the mean ± standard error of the mean (SEM). A p value <0.05 was considered significant (SSPS Statistical Program, version 15.0.; SPSS Inc, Chicago, IL, USA).
Results
Increased presence of hsp60 in skin biopsies of atopic dermatitis
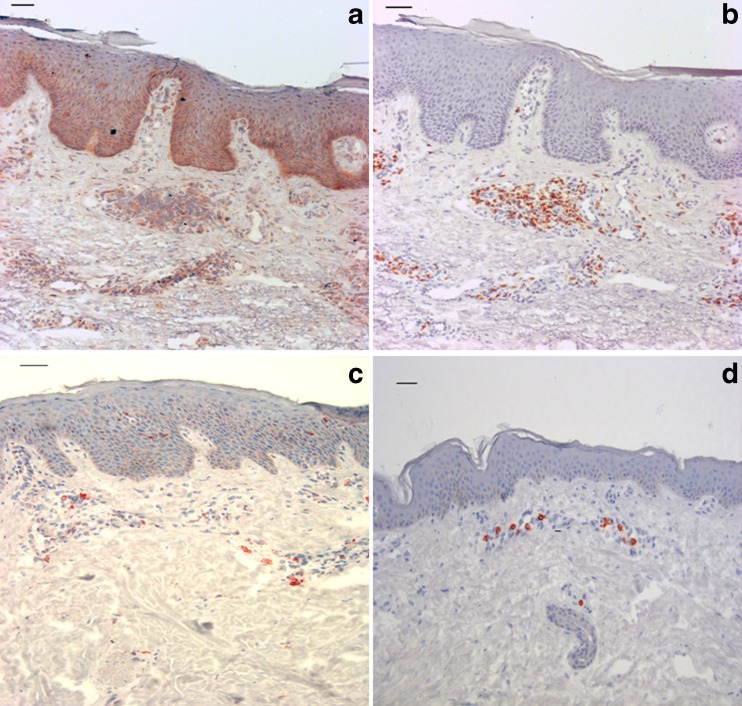
Since hsp are upregulated in inflammatory disorders, we questioned whether the presence of hsp60 is increased in the lesional AD skin (Brusko et al. 2008). To demonstrate this, skin biopsies from both nonlesional and lesional skin sites from AD patients were stained for hsp60 and CD3. The results of a representative donor are shown in Fig. 1. The presence of hsp60 was seen in both the healthy skin and the AD skin, but the pattern of hsp60 presence was strikingly different. The dermis and epidermis of atopic skin was characterized by large cell infiltrates, with the presence of hsp60, which was not seen to that extent in the healthy skin. Staining for CD3 in sequential slides was indicative for the presence of hsp60 in CD3+ cells in the dermis. However, since it was not a double staining, we cannot exclude that, next to T cells, hsp60 is also localized in other cells or extracellularly in dermal tissue, in close proximity to CD3 cells. These data show that hsp60 is highly present in inflamed tissue of patients with AD.
Fig. 1.
Human hsp60 is expressed in the skin. a hsp60 staining in an active lesion of a patient with AD. b The same skin of the patient with AD, colored with anti-CD3. Both are representative pictures of skin biopsies of an active skin lesion of a patient with AD. Compared to the nonlesional skin (c and d), the thickened stratum corneum and epidermis are typical for AD. In the dermis, infiltration of cells is seen. hsp60 is mostly seen in these infiltrates and in the lower layers of the epidermis (a), whereas coloring grows less dense in the upper areas of the epidermis. The cell infiltrates express CD3 and the hsp60 expression in the dermis seems confined to these cells. c and d Nonlesional skin of a patient with AD. Compared to the lesional skin, the nonlesional skin shows less hsp60 (c) and CD3 (d). ×200 (scale bar = 50 μm)
Human hsp60-specific proliferation
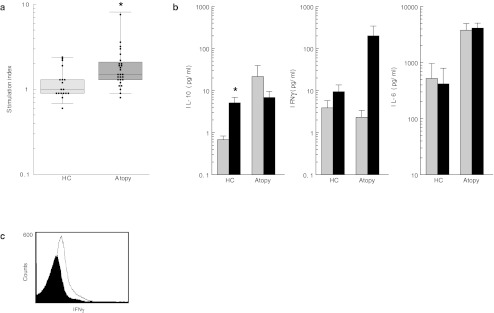
Next, we wanted to see whether this increased recognition of hsp60 is also seen in peripheral T cell of children with AD. To address this question, proliferation of PBMC was determined in response to hsp60. Proliferative responses of 28 patients were compared with those of 18 HC. The SI of the PBMC was significantly higher in the patient group compared to the HC group (p = 0.004). This indicates that PBMC from children with AD proliferate more to hsp60 compared to PBMC from HC (Fig. 2a), whereas no significant difference was found on a control mitogen (Supplementary Fig. 1).
Fig. 2.
Human hsp60 is recognized by PBMC of atopic infants and induce cytokine secretion. a Protein-induced T cell proliferation in atopic children and HC. PBMC were cultured for 96 h in the presence of human hsp60. Boxes show IQR for each group of data, horizontal lines show the median. *p < 0.05, significant differences in responses. Light grey boxes HC, dark grey boxes atopic children. SI is indicated on a logarithmic scale. b Protein-induced cytokine secretion of IL-10, IFNγ, and IL-6 in atopic children and HC. Boxes show the mean levels of cytokine with SEM. White boxes medium, black boxes stimulation with hsp60. Cytokine levels are indicated on a logarithmic scale. c FACS analysis of CD4+CD14− T cells from an atopic donor stimulated with hsp60 for 4 days and stained for IFNγ. Histogram shows the medium level (black) and IFNγ level (white) after stimulation with hsp60
Human hsp60-specific cytokine induction
Given the increased T cell proliferation to hsp60 by the T cells of atopic children, we wanted to assess the quality of T cell activation. First, cytokine production by hsp60-induced PBMC was measured in culture medium of 26 patients and 22 HC. When compared to the medium, stimulation with hsp60 induced a significantly higher level of IL-10 in the HC group (mean ± SEM, 4.4 ± 1.8 pg/ml; p = 0.012), while in the patient group, compared to the medium, production of IL-10 was lower after stimulation with hsp60 (mean ± SEM, −14.77 ± 17.91 pg/ml; ns). A different pattern was seen for IFNγ. Following in vitro stimulation with hsp60, PBMC from both patients (mean ± SEM, 198.1 ± 140.1) and HC (mean ± SEM, 5.58 ± 4.46) produced higher amounts of IFNγ. Taken together, these results demonstrate that stimulating PBMC from atopic patients with hsp60 leads to the induction of IFNγ but not IL-10 (Fig. 2). Of the other cytokines tested (IL-4, IL-5, IL-12, and IL-13), only IL-6 showed an increase after stimulation with hsp60 (Fig. 2b).
Although the LPS content of the hsp60 used was below the detection level and although monocytes are not expected to survive after a 5-day culture, we wanted to verify that IFNγ measured was indeed secreted by T cells. Therefore, after culturing the cells with or without hsp60, cells were stained for CD4, CD14, and IFNγ. Indeed, hsp60-induced production of IFNγ was produced by CD4+CD14− T cells, while virtually no monocytes (<1 %) were present. Figure 2c shows a representative overlay histogram of CD4+CD14−IFNγ+ cells.
Human hsp60-specific induction of CD4+CD25Bright T cells expressing the transcription factor FOXP3
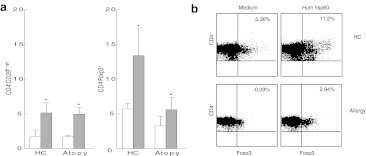
We next questioned whether in atopic children in vitro activation with hsp60 could lead to the induction of Tregs. To address this issue, we first investigated whether the FOXP3 expression in hsp60-specific CD4+ T cells was different between HC and atopic children. PBMC of five HC and nine children with AD were cultured for 7 days in vitro with hsp60 (see Fig. 3). Stimulation with hsp60 gave a significantly higher percentage of CD4+CD25Bright T cells and CD4+FOXP3+ T cells in both the HC and the atopic patients compared to the medium only (see Fig. 3a, b and Table 2). Although at least a part of the FOXP3 induction is due to the activation of T cells, stimulation with hsp60 also seems to induce T cells with the phenotypical characteristics of Tregs, expressing CD25 and FOXP3.
Fig. 3.
Human hsp60 induces CD4+CD25bright T cells and the expression of FOXP3. Cells were cultured in human hsp60 for 7 days. a FACS analysis shows a significantly higher level of CD4+CD25bright T cells and CD4+FOXP3+ T cells in both HC as atopic individuals. White boxes medium only, grey boxes human hsp60. b FACS analysis of CD4+ T cells of both atopic individuals and HC expressing FOXP3 after stimulation with either medium or human hsp60. Percentages indicate the percentage of CD4+FOXP3+T cells in the total CD4+ T cell population measured
Table 2.
Populations of CD4+CD25bright and CD4+FOXP3+ T cells increase after stimulation with hsp60
| Medium | Human hsp60 | p value | ||
|---|---|---|---|---|
| HC | CD4+CD25Bright | 1.69 ± 0.72 | 5.13 ± 3.29 | <0.05 |
| CD4+FOXP3+ | 5.69 ± 1.73 | 13.37 ± 8.65 | <0.05 | |
| Atopic patients | CD4+CD25Bright | 1.67 ± 0.21 | 4.95 ± 0.97 | <0.001 |
| CD4+FOXP3+ | 3.25 ± 1.39 | 5.58 ± 1.78 | <0.05 | |
This table shows the mean percentages ± SEM for the different cell populations in FACS analysis after stimulation with human hsp60
Human hsp60-induced CD4+CD25+CD127− T cells are not suppressive in vitro
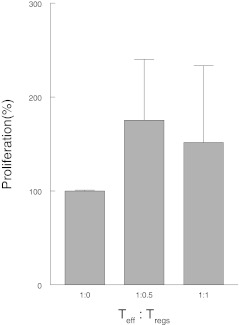
Since CD4+ T cells responding to hsp60 produce IFNγ while also sharing phenotypical characteristics of Tregs, we questioned whether these hsp60-induced CD4+CD25+FOXP3+ are functional Tregs, with the capacity to suppress T effector cells. To evaluate the functionality of these induced CD4+CD25+FOXP3 T cells, suppression assays were performed on PBMC of six children with AD. Freshly sorted CD4+CD25− T effector cells were cocultured with CD4+CD25+CD127− T cells obtained from PBMC precultured for 4 days with hsp60 (see Fig. 4). Despite high levels of FOXP3, the CD4+CD25+CD127− T cells from these cultures were not suppressive in five of six patients. In only one patient, the cells were able to suppress effector cells. In comparison, in all HC, these cells are suppressive in this classical suppression assay (Vercoulen et al. 2009). These findings indicate that, even though hsp60, presented in an atopic environment, induces T cells that resemble Tregs phenotypically, these cells are not capable of suppressing effector T cells in vitro.
Fig. 4.
Human hsp60-induced CD4+CD25+CD127− T cells from atopic patients have no suppressive function. Functional assays were performed as described in the “Methods” section. The figure shows the percentage difference in proliferative response between CD4+CD25− T cells (Teff) cultured alone and CD4+CD25− T cells cultured in the presence of induced CD4+CD25+CD127− T cells (Tregs) in three different ratios. Only one patient showed suppressive function
Discussion
In the present study, we demonstrated for the first time the increased presence of hsp60 in the skin of patients with AD. Furthermore, we found increased proliferation of T cells from atopic children after in vitro stimulation. Both increased levels of hsp60 at the site of inflammation and the enhanced T cell response underline that hsp60 may act as the target of an (autoantigenic) T cell response. The next question was what type of T cell response was induced by hsp60 in AD. At first glance, it seemed that hsp-induced T cells had a regulatory phenotype as they expressed CD25bright and FOXP3. However, despite the expression of FOXP3, hsp60-induced CD4+CD25+CD127− cells of AD patients were not functionally suppressive in vitro. Thus, these cells are most probably not Tregs but highly activated effector T cells. The presence of activated but not Tregs may be in line with previous studies showing that, in active atopic diseases, the suppressive function of Tregs is diminished (Ling et al. 2004; Tiemessen et al. 2004). Interestingly, a recent study by Wehrens et al. revealed that highly activated T effector cells are resistant to suppression through PKB/c-akt hyperactivation. This might also be an explanation why these phenotypically regulatory cells fail to suppress T effector cells in vitro and needs further investigation (Wehrens et al. 2011).
In atopic diseases, it has been speculated whether specific targeting of T cells could provide a novel immunomodulatory pathway for treatment. Up till now, studies have focused on the use of synthetic peptides which contain T cell epitopes derived from known allergens, such as Fel d 1 (cat allergy) and Api m 1 (bee venom) (Muller et al. 1998; Pene et al. 1998). However, since atopic individuals are mostly polysensitized, immunotherapy aimed at one allergen might have limited effect in the majority of the patients.
Another possibility for immunomodulation is the use of bystander antigens, in which the antigen used is not the causative antigen, but an antigen which can alter the reaction by either bystander activation or suppression (Larche et al. 2006). Prerequisites for bystander epitopes are expression at the site of inflammation and recognition by immune-competent cells such as T cells. For this type of antigen-specific targeting of T cells, hsp60 is a potential candidate (van Eden et al. 2005; Zanin-Zhorov et al. 2006), as hsp60 was previously described in skin lesions of patients with Behçet’s disease (Ergun et al. 2001). hsp60 has also been described as a target for T cells in TH1 diseases such as diabetes mellitus and juvenile idiopathic arthritis and as a target for T cells in the inflammatory process contributing to atherosclerotic lesions (Albani et al. 1995; Grundtman et al. 2011; Prakken et al. 2004; Raz et al. 2001).
It has to be noted, though, that hsp60 primarily acts as an intracellular (and intramitochondrial) chaperone. Only in particular conditions, such as cell stress, hsp60 will be released into the extracellular environment, where it may have “extra-chaperoning” roles, including interaction with immune system cells (Macario et al. 2010; Pockley and Multhoff 2008). This secretion of hsp60 may originate either from lymphocytes themselves, from other cutaneous cells like keratinocytes and fibroblasts, or from other sources such as nerves or vessels (De Maio 2011). Also, next to hsp60, other proteins involved in inflammation, including proteins from other hsp families, may be involved in AD pathogenesis (Ghoreishi et al. 2000; Ghoreishi 2000; Ishibashi et al. 2009; Matsumoto et al. 2002; Park et al. 2008).
This is the first study to demonstrate that hsp60 is present at the site of inflammation and that it induces specific proinflammatory T cell responses in children with AD. This is in line with a previous experimental study showing that hsp60 can induce cytokines of a regulatory and TH1 phenotype in the skin of dogs with AD (Jassies-van der Lee et al. 2008). This finding is an important condition in investigating novel targets for immunomodulation. Further studies are needed to investigate these immunomodulating properties of hsp60 in atopic diseases.
Electronic supplementary material
Protein-induced T cell proliferation in atopic children and healthy controls. PBMCs were cultured for 96 hours in the presence of ConA. Boxes show IQR for each group of data, horizontal lines show the median. Light grey boxes = healthy controls, dark grey boxes = atopic children. SI is indicated on a logarithmic scale. The difference in SI between the HC and atopic children was not significant (p = 0.1) after stimulation with ConA (JPEG 19 kb)
Glossary
- TH2
T helper cells type 2
- TH1
T helper cells type 1
- Tregs
Naturally occurring or induced CD4+CD25+FOXP3+ regulatory T cells
- hsp
Heat shock protein(s)
- hsp60
Human heat shock protein 60
- PBMC
Peripheral blood mononuclear cells
- AD
Atopic dermatitis
- FACS
Fluorescence-activated cell sorting
References
- Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123(4):735–746. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Albani S, Keystone EC, Nelson JL, Ollier WE, La CA, Montemayor AC, Weber DA, Montecucco C, Martini A, Carson DA. Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nat Med. 1995;1(5):448–452. doi: 10.1038/nm0595-448. [DOI] [PubMed] [Google Scholar]
- Albani S, Koffeman EC, Prakken B. Induction of immune tolerance in the treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2011;7(5):272–281. doi: 10.1038/nrrheum.2011.36. [DOI] [PubMed] [Google Scholar]
- Alexander C, Ying S, Kay B, Larche M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+; CD4+ interferon-gamma+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35(1):52–58. doi: 10.1111/j.1365-2222.2005.02143.x. [DOI] [PubMed] [Google Scholar]
- Bellinghausen I, Klostermann B, Knop J, Saloga J. Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J Allergy Clin Immunol. 2003;111(4):862–868. doi: 10.1067/mai.2003.1412. [DOI] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Jager W, Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10(1):133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager W, Prakken BJ, Bijlsma JW, Kuis W, Rijkers GT. Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J Immunol Methods. 2005;300(1–2):124–135. doi: 10.1016/j.jim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Kleer IM, Kamphuis SM, Rijkers GT, Scholtens L, Gordon G, Jager W, Hafner R, Zee R, Eden W, Kuis W, Prakken BJ. The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat-shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum. 2003;48(7):2001–2010. doi: 10.1002/art.11174. [DOI] [PubMed] [Google Scholar]
- Kleer I, Vercoulen Y, Klein M, Meerding J, Albani S, Zee R, Sawitzki B, Hamann A, Kuis W, Prakken B. CD30 discriminates heat shock protein 60-induced FOXP3+ CD4+ T cells with a regulatory phenotype. J Immunol. 2010;185(4):2071–2079. doi: 10.4049/jimmunol.0901901. [DOI] [PubMed] [Google Scholar]
- Maio MA. Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16(3):235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergun T, Ince U, Eksioglu-Demiralp E, Direskeneli H, Gurbuz O, Gurses L, Aker F, Akoglu T. HSP 60 expression in mucocutaneous lesions of Behcet’s disease. J Am Acad Dermatol. 2001;45(6):904–909. doi: 10.1067/mjd.2001.117728. [DOI] [PubMed] [Google Scholar]
- Ghoreishi M. Heat shock proteins in the pathogenesis of inflammatory skin diseases. J Med Dent Sci. 2000;47(2):143–150. [PubMed] [Google Scholar]
- Ghoreishi M, Yokozeki H, Hua WM, Nishioka K. Expression of 27 KD, 65 KD and 72/73 KD heat shock protein in atopic dermatitis: comparison with those in normal skin and contact dermatitis. J Dermatol. 2000;27(6):370–379. doi: 10.1111/j.1346-8138.2000.tb02186.x. [DOI] [PubMed] [Google Scholar]
- Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(5):960–968. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guajardo JR, Schleifer KW, Daines MO, Ruddy RM, Aronow BJ, Wills-Karp M, Hershey GK. Altered gene expression profiles in nasal respiratory epithelium reflect stable versus acute childhood asthma. J Allergy Clin Immunol. 2005;115(2):243–251. doi: 10.1016/j.jaci.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y, Kato H, Asahi Y, Sugita T, Nishikawa A. Identification of the major allergen of Malassezia globosa relevant for atopic dermatitis. J Dermatol Sci. 2009;55(3):185–192. doi: 10.1016/j.jdermsci.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Jassies-van der Lee A, Rutten V, Kooten P, Zee R, Willemse T. Intradermal injection of Hsp60 induces cytokine responses in canine atopic and healthy skin. Cell Stress Chaperones. 2008;13(3):387–391. doi: 10.1007/s12192-008-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis S, Kuis W, Jager W, Teklenburg G, Massa M, Gordon G, Boerhof M, Rijkers GT, Uiterwaal CS, Otten HG, Sette A, Albani S, Prakken BJ. Tolerogenic immune responses to novel T-cell epitopes from heat-shock protein 60 in juvenile idiopathic arthritis. Lancet. 2005;366(9479):50–56. doi: 10.1016/S0140-6736(05)66827-4. [DOI] [PubMed] [Google Scholar]
- Kapitein B, Hoekstra MO, Nijhuis EHJ, Hijnen DJ, Arets HG, Kimpen JL, Knol EF. Gene expression profiles in CD4+ T cells reflects heterogeneity in infant wheezing phenotypes. Eur Respir J. 2008;32(5):1203–1212. doi: 10.1183/09031936.00020108. [DOI] [PubMed] [Google Scholar]
- Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6(10):761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363(9409):608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- Macario AJ, Cappello F, Zummo G, Conway ME. Chaperonopathies of senescence and the scrambling of interactions between the chaperoning and the immune systems. Ann N Y Acad Sci. 2010;1197:85–93. doi: 10.1111/j.1749-6632.2010.05187.x. [DOI] [PubMed] [Google Scholar]
- Madore AM, Perron S, Turmel V, Laviolette M, Bissonnette EY, Laprise C. Alveolar macrophages in allergic asthma: an expression signature characterized by heat shock protein pathways. Hum Immunol. 2010;71(2):144–150. doi: 10.1016/j.humimm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Oshida T, Obayashi I, Imai Y, Matsui K, Yoshida NL, Nagata N, Ogawa K, Obayashi M, Kashiwabara T, Gunji S, Nagasu T, Sugita Y, Tanaka T, Tsujimoto G, Katsunuma T, Akasawa A, Saito H. Identification of highly expressed genes in peripheral blood T cells from patients with atopic dermatitis. Int Arch Allergy Immunol. 2002;129(4):327–340. doi: 10.1159/000067589. [DOI] [PubMed] [Google Scholar]
- Muller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, Blaser K. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101(6 Pt 1):747–754. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- Park YD, Park D, Bhak J, Yang JM. Proteomic approaches to the analysis of atopic dermatitis and new insights from interactomics. Proteomics Clin Appl. 2008;2(3):290–300. doi: 10.1002/prca.200780063. [DOI] [PubMed] [Google Scholar]
- Pene J, Desroches A, Paradis L, Lebel B, Farce M, Nicodemus CF, Yssel H, Bousquet J. Immunotherapy with Fel d 1 peptides decreases IL-4 release by peripheral blood T cells of patients allergic to cats. J Allergy Clin Immunol. 1998;102(4 Pt 1):571–578. doi: 10.1016/S0091-6749(98)70294-5. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Multhoff G. Cell stress proteins in extracellular fluids: friend or foe? Novartis Found Symp. 2008;291:86–95. doi: 10.1002/9780470754030.ch7. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Samodal R, Le TD, Giannoni F, Yung GP, Scavulli J, Amox D, Roord S, Kleer I, Bonnin D, Lanza P, Berry C, Massa M, Billetta R, Albani S. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101(12):4228–4233. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajka G. Essential aspects of atopic dermatitis. Berlin: Springer; 1989. [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358(9295):1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- Roord ST, Zonneveld-Huijssoon E, Le T, Yung GP, Koffeman E, Ronaghy A, Ghahramani N, Lanza P, Billetta R, Prakken BJ, Albani S. Modulation of T cell function by combination of epitope specific and low dose anticytokine therapy controls autoimmune arthritis. PLoS One. 2006;1:e87. doi: 10.1371/journal.pone.0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen MM, Ieperen-Van Dijk AG, Bruijnzeel-Koomen CA, Garssen J, Knol EF, Hoffen E. Cow’s milk-specific T-cell reactivity of children with and without persistent cow’s milk allergy: key role for IL-10. J Allergy Clin Immunol. 2004;113(5):932–939. doi: 10.1016/j.jaci.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Umetsu DT, Akbari O, Dekruyff RH. Regulatory T cells control the development of allergic disease and asthma. J Allergy Clin Immunol. 2003;112(3):480–487. doi: 10.1016/S0091-6749(03)01869-4. [DOI] [PubMed] [Google Scholar]
- Eden W, Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5(4):318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- Eden W, Spiering R, Broere F, Zee R. A case of mistaken identity: HSPs are no DAMPs but DAMPERs. Cell Stress Chaperones. 2012;17(3):281–292. doi: 10.1007/s12192-011-0311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercoulen Y, Wehrens EJ, Teijlingen NH, Jager W, Beekman JM, Prakken BJ. Human regulatory T cell suppressive function is independent of apoptosis induction in activated effector T cells. PLoS One. 2009;4(9):e7183. doi: 10.1371/journal.pone.0007183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens EJ, Mijnheer G, Duurland CL, Klein M, Meerding J, Loosdrecht J, Jager W, Sawitzki B, Coffer PJ, Vastert B, Prakken BJ, Wijk F. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells. Blood. 2011;118(13):3538–3548. doi: 10.1182/blood-2010-12-328187. [DOI] [PubMed] [Google Scholar]
- Werfel T, Wittmann M. Regulatory role of T lymphocytes in atopic dermatitis. Chem Immunol Allergy. 2008;94:101–111. doi: 10.1159/000154935. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116(7):2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein-induced T cell proliferation in atopic children and healthy controls. PBMCs were cultured for 96 hours in the presence of ConA. Boxes show IQR for each group of data, horizontal lines show the median. Light grey boxes = healthy controls, dark grey boxes = atopic children. SI is indicated on a logarithmic scale. The difference in SI between the HC and atopic children was not significant (p = 0.1) after stimulation with ConA (JPEG 19 kb)