Abstract
Intraarterial chemotherapy (IAC) is considered effective for the treatment of solid tumors with high local doses of systemically toxic chemotherapeutics. Osteosarcoma, which is often located in the extremities, is a potential target for IAC. However, the efficacy of this treatment modality has varied, and standardized protocols are difficult to establish due to tumor heterogeneity and the limited numbers of patients available for clinical trials. Reproducible experimental models are needed to further investigate IAC in osteosarcoma. Here, we describe a new microsurgical technique for repeated infusion of drugs into the mouse femoral artery for local treatment of experimental intratibial metastasizing osteosarcoma. We successfully achieved 5 catheterizations at 3-d intervals in 70% of the mice tested. Laser speckle imaging indicated a maximal 50% reduction in blood flow around the ankle region of catheterized legs infused with 0.5 mg/kg cisplatin. However, blood flow in the front feet was affected only minimally. Histologic examination of catheterized arteries of saline control or cisplatin-treated mice showed circular fibrinoid media necrosis and partial thrombosis, but total occlusion of the arteries was not observed. The method we describe for repeated transient catheterization of the mouse femoral artery likely will be useful in future studies comparing the efficacies of intraarterial and systemic cisplatin treatment of intratibial metastasizing osteosarcoma in mice under standardized conditions.
Abbreviation: IAC, intraarterial chemotherapy
Intraarterial administration of drugs for local treatment of diseased tissue was first described more than 100 y ago for the treatment of local infections.18 Repeated injections of methyl bis amine hydrochloride into the nutrient artery as a method for cancer therapy was first described in 1950.12 Intraarterial chemotherapy (IAC) was investigated in several clinical trials for the treatment of liver metastases in primary uveal melanoma, mammary carcinoma, intracranial neoplasms, retinoblastoma, and head and neck cancer but did not significantly increase long-term survival of the patients.8,13,14,17,19,20,21,22,25 Nevertheless, IAC has been approved for clinical use as a local tumor therapy.
In the 1980s, IAC was introduced for the treatment of osteosarcoma. Pharmacologic studies with cisplatin, which is frequently used in osteosarcoma chemotherapy, showed that local administration through the main artery feeding the tumor resulted in high local drug concentrations and, consequently, an improved first-pass effect on tumor tissue compared with that after systemic administration.4,26 In additional studies during the last 30 y, different protocols have been used to further increase the efficacy of IAC compared with systemic treatment of osteosarcoma, but the results remained inconsistent and were frequently inconclusive.2,9,11 Nevertheless, a recent study revealed a remarkably increased 5-y survival rate of more than 90% for patients with localized osteosarcoma who received combined intraarterial–intravenous therapy with cisplatin and doxorubicin.27 Reasons for the observed heterogeneity in the results from clinical trials of IAC for osteosarcoma include the heterogeneity of primary tumors, lack of standardized protocols, and the small number of patients due to the low incidence of osteosarcoma. These drawbacks, together with the logistically and technically demanding procedures for IAC, have retarded further progress in the contexts of osteosarcoma and head and neck cancer.3 Consequently, a rat model involving the infusion of the external carotid artery with single- and multiple-drug regimens has been established and used under standardized experimental conditions for head and neck squamous cell carcinoma.23 This cited work23 demonstrated that IAC with bleomycin and 5-fluorouracil was superior to systemic treatment regimens. These results stimulated research on IAC in head and neck cancer and ultimately led to the development of the promising RadPlat protocol.1
Studies in small rodents have established methods for continuous catheterization of the jugular vein for drug administration and blood sampling in rats and mice.15,16 The present study aimed at developing a simple and easily reproducible technique for repeated catheterization and subsequent drug infusion through the femoral artery of mice, avoiding the main disadvantages of permanent catheterization, occlusion of the femoral artery and nonphysiologic perfusion of the periphery.10,15 Mice are particularly attractive as animal models for experimental osteosarcoma because of their minimal requirements for space and housing and the availability of multiple models with different genetic backgrounds. The current results demonstrate the feasibility of repeated catheterization (maximum, 5 infusions at 3-d intervals) of the femoral artery of mice for rapid manual infusion of cisplatin for the treatment of intratibial osteosarcoma under standardized conditions.
Materials and Methods
Animals.
Female C3H/HeNCrl (C3H) mice (weight, 24 to 26 g) were obtained from Charles River Laboratories (Sulzfeld, Germany) at least 7 d prior to beginning the experiments. Housing conditions and experimental protocols were in accordance with institutional guidelines and approved by the Cantonal Veterinary Department (Zurich, Switzerland). The mice were housed in groups of 5 in 32 × 16 cm cages at room temperature under natural conditions of lighting and humidity.
Glass capillaries and catheters.
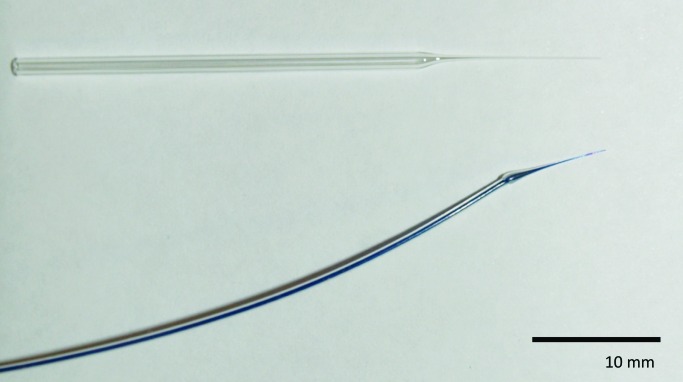
Glass capillaries (Bio Medical Instruments, Zöllnitz, Germany) with an outer diameter of 1.2 mm were heated and pulled with a standard puller (Sutter Instrument, Basel, Switzerland) to obtain a cone-shaped tip with an outer diameter of approximately 100 μm (Figure 1). Catheters were made from polyethylene tubing (inner diameter, 0.28 mm; outer diameter, 0.61 mm; Portex, Smiths Medical, Ashford, UK). The tubing was heated with a soldering iron, adjusted to 400 °C and pulled until a cone-shaped tip was obtained. Catheter tips were inspected under a stereo microscope (magnification, 31.5×; SZX 10, Olympus Schweiz, Volketswil, Switzerland) and the diameters measured by using a stage micrometer (Nikon, Egg, Switzerland). The tips of the catheters used had an outer diameter of 110 µm ± 30 µm (n = 44) and were angled by using a scalpel. Capillaries and catheters were flushed with 70% ethanol and then with sterile 0.9% NaCl. Needles attached to 0.5-mL syringes (29 gauge × 12.7 mm, BD Micro-Fine, BD Medical, Heidelberg, Germany), used as a reservoir, were blunted and connected to the polyethylene catheters.
Figure 1.
Small cone-shaped glass capillary used for puncturing the femoral artery (top) and polyethylene catheter (outer diameter, approximately 110 μm) with a cone-shaped tip used for drug infusions.
Anesthesia.
Mice were anesthetized by using 5% isoflurane (Forene, Abbott, Baar, Switzerland) in oxygen (300 mL/min) in a 12 × 9 × 8 cm chamber (custom-made from a pipette-tip box) connected to a vaporizer (VIP 300, Matrix, Crans-Montana, Switzerland). Anesthetized mice were laid on their backs on a heating pad (Horn, Gottmadingen, Germany), and the body temperature was maintained at 37 °C. The head of each mouse was placed in a truncated 10-mL syringe used as a face mask that was connected to the vaporizer by use of a silicone tube. During surgery, anesthesia was maintained by using an inspiratory isoflurane concentration of 2% to 3%.
Surgery and drug infusion.
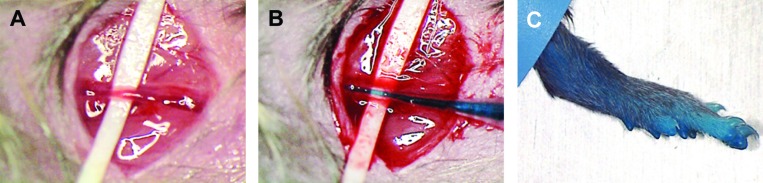
The health status of the mice was carefully checked before each surgical intervention and included brief observation of the behavior of the animals in their cages. Mice were weighed and underwent wound inspection for signs of inflammation (for example, pus or perifocal erythema) while anesthetized. Mice treated with cisplatin were weighed daily. Weight loss throughout the entire experiment was less than 10% in all mice investigated. An eye ointment (retinoli palmitas 15000 IU; Bausch and Lomb, Zug, Switzerland) was applied to prevent corneal dehydration during surgery. The hindlimbs were fixed with tape in slight abduction, and the inguinal area of the left leg was shaved, disinfected with 70% ethanol, and exposed for surgery. Surgery was performed under a stereo microscope (magnification, 31.5×; SZX 10, Olympus). The femoral artery, vein, and nerve were exposed through a skin incision of 3 to 4 mm parallel and inferior to the inguinal ligament. The femoral artery was separated from surrounding tissues, and a strip (width, 2 mm; length, 10 to 15 mm) of latex excised from a latex glove and disinfected with 70% ethanol was placed underneath the vessel (Figure 2 A). A small hole was created in the artery approximately 2 to 4 mm distally the intersection with the inguinal ligament by using a custom cone-shaped glass capillary tube. The small hole in the vessel wall was enlarged by progressive insertion of the cone-shaped capillary. The glass capillary was replaced with the polyethylene tubing by advancing the thinned tip into the artery (Figure 2 B).
Figure 2.
Catheterization of the femoral artery. The femoral artery was exposed through a skin incision of 3 to 4 mm, (A) a latex strip was laid underneath the artery, and (B) a polyethylene catheter was inserted. (C) Blue staining of the operated leg after infusion of isosulfan blue indicated successful catheterization of the femoral artery.
Free flow through the catheter was confirmed through manual infusion within 2 min (timed by using a stop watch) of 350 μL of a solution of 0.7% isosulfan blue (Kantonsapotheke Zurich, Zurich, Switzerland) in 0.9% saline (B Braun Medical, Sempach, Switzerland). The same procedure and vehicle were used when cisplatin (0.5 mg/kg body weight; Ebewe Pharma-Sandoz, Holzkirchen, Germany) was infused. Infusions were considered successful when the limb distal to the inserted catheter turned blue within seconds after administration of isosulfan blue (Figure 2 C). Systemic distribution of infused compounds was indicated by a faint blue appearance of the entire animal within minutes after infusion. After completion of the infusion, the wound was flushed with 1 mL 0.9% saline, the catheter and latex strip were removed, and the incision was closed (7-0 silk, B Braun Medical). Finally, slight compression was applied to the wound for approximately 3 min to prevent bleeding. Once mice had recovered from surgery, they were put back into their cages and observed for social behavior, food intake, and hygienic behavior over a period of approximately 4 h. All mice showed normal social behavior throughout the experiment, and postsurgical analgesia was not needed. To avoid wound infection, bedding was changed immediately after surgery and then every second day. Bedding compounds and cages were autoclaved. Mice removed the skin sutures within 1 d, but infection did not occur. Fibrin clots were removed immediately before the next intervention by using a small forceps moistened with 0.9% saline, thus allowing repeat surgery through the same incision. Occasionally some scar tissue had to be removed to achieve access.
Measurement of blood flow.
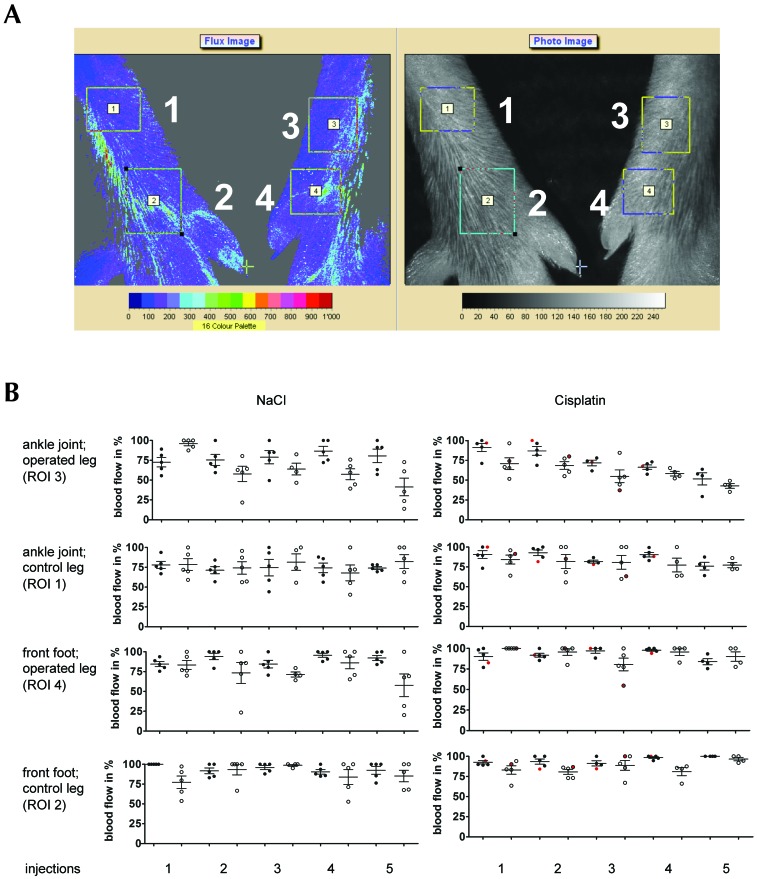
Blood flow was recorded by using laser speckle contrast imaging to measure superficial capillary perfusion to a tissue depth of 200 to 300 µm (Moor FLPI, Moor Instruments, Axminster, UK). Blood flow was measured in regions of interest (ROI) around the ankle joint and the front foot area of the operated and nonoperated (control) legs of individual mice (Figure 3 A) just before and 10 min after surgical intervention and infusion of saline (control mice) or cisplatin. All measurements were performed by the surgeon and assisting surgeon. Blood flow recordings (duration, 4 ms) were collected every 4 s for 2 min. The highest blood flow value recorded in an individual mouse at any ROI during a single intervention was set to 100% (reference value), and all other values recorded at that time point from the various ROI were normalized to this reference value. The normalized values in each ROI were averaged to obtain the mean blood flow in that ROI in that mouse at that time point. This calculation was done for each mouse and time point of blood flow measurement.
Figure 3.
Blood flow in mouse hindlimbs before and 10 min after repeated catheterization of the femoral artery and infusion of sodium chloride (control mice) or cisplatin at 3-d intervals. (A) Representative images of blood flow (left) and photo (right) of control (left) and catheterized (right) legs in indicated regions of interest around the ankle (1, 3) and forefoot (2, 4). (B) Mean (± 1 SD) and single blood flow measurements in percentage of highest value recorded in mice before (filled circles) and 10 min after (open circles) catheterization and repeated infusion (maximum, 5) of NaCl (left) or cisplatin (right). The single values obtained from a cisplatin-treated mouse that had only 3 successful catheterizations are shown in red.
Histology.
After the first, third, and fifth catheterizations, representative mice were euthanized by anesthesia with 5% isoflurane and subsequent atlantoaxial dislocation. Both hindlegs were disarticulated at the hip. The quadriceps femoris muscle, femoral vessels, and the femoral nerve were detached from the leg. A specimen of the limb between the inguinal ligament and a site 3 to 4 mm distal to the injection site was dissected and fixed in 10% formalin in PBS and embedded in paraffin. Tissue cross-sections (thickness, 6 µm) at intervals of 200 µm were stained with hematoxylin and eosin. Ten cross-sections per limb specimen were analyzed, and images (magnification, 10× and 20×) of representative tissue areas were taken by using a digital camera (AxioCam MRc, Carl Zeiss MicroImaging, Feldbach, Switzerland) attached to an inverted microscope (AxioObserver Z1, Zeiss).
Results
Catheterization of the mouse femoral artery.
We designed a pilot study involving 10 mice to determine the success rate after 5 consecutive catheterizations (3 d between sequential catheterizations) of the femoral artery at the same site, with the aim to establish a technique for hindlimb IAC in mouse models of intratibial osteosarcoma. Catheterization was considered successful when the limb distal to the site of isosulfan blue infusion appeared blue within seconds after injection (Figure 2 C). According to this criterion, we achieved 2 successful catheterizations in all mice, except for one animal that died of unknown causes during surgery. Moreover, at least 3 consecutive catheterizations were successful in 8 mice and 5 interventions in 7 of 10 mice, equivalent to a success rate of 70%. None of the 9 mice showed any signs of pain, including restricted use of the operated leg and self-mutilation, even though postoperative analgesics were not provided. Within 10 min after surgery, the mice showed normal social behavior, grooming, and eating. Successfully catheterized arteries developed an aneurysm at the injection site that was easy to reopen during the next intervention. In rare cases of difficult injections due to vessel constriction before appropriate insertion of the catheter or to a suboptimal shape of the custom catheter, scarring of the surrounding tissue with consecutive obliteration of the vessel aggravated later catheterizations. However, intravascular clotting was not observed.
Function and histology of the mouse femoral artery after repeated catheterization and infusion of saline or cisplatin.
The results of the pilot study showed the feasibility of repeated catheterization of the femoral artery for 5 interventions at 3-d intervals in individual mice. We then sought to investigate the effects of the surgical manipulations and of repeated infusion of saline or the chemotherapeutic agent cisplatin on perfusion distal to the site of catheterization and on vessel morphology. To this end, we infused each of 9 mice with 350 μL of 0.9% saline containing 0.7% isosulfan blue (control mice) and 5 mice with cisplatin (0.5 mg/kg body weight) in 350 μL 0.9% saline, 0.7% isosulfan blue. Perfusion was measured by laser speckle contrast imaging immediately before and 10 min after surgery and infusion. In addition, we histologically evaluated the femoral arteries of control nonoperated legs and of catheterized legs in 2 control mice that each underwent a single catheterization and in 2 additional control mice that underwent 3 interventions. The remaining 5 control mice and the 5 cisplatin-treated animals were analyzed after 5 catheterizations.
Measurements of perfusion in noncatheterized control legs around the ankle joint (ROI 1, Figure 3 A) and front foot (ROI 2) were performed in all mice investigated to assess the normal range of fluctuation. The mean values of perfusion in distinct ROI of control legs, averaged from all mice in each group at each time point, varied by less than 10% when the mean values at all time points were compared (Figure 3 B). However, the normalized perfusion values in a specific ROI of the control leg of individual mice within a particular group at a particular time point differed by as much as 50%.
Perfusion measurements distal to the catheterized artery in legs of mice that received saline indicated decreases in mean blood flow around the ankle after as compared with before the interventions, except for the first series of catheterizations in this group of mice (Figure 3 B). The expected intervention-related decrease in mean perfusion around the ankle joint in catheterized legs changed progressively from 57.8% (range, 21.7% to 80.4%; n = 5) after the second catheterization to 41.3% (range, 13.8% to 72.7%; n = 5) after the fifth catheterization. However, perfusion in the operated legs fully recovered during the intervention-free 3-d period between each catheterization for the full 5-infusion series. The decrease in perfusion in the front foot area of operated legs was qualitatively less pronounced than that around the ankle joint and, much like in the ankle joint area, recovered after each intervention to the mean perfusion level observed prior to the first catheterization.
Different results were obtained in mice that received infusions of cisplatin through the catheter in the femoral artery. For these mice, the mean superficial perfusion in the ankle joint area of the operated leg decreased progressively from 70.8% (range, 96.4% to 51.6%; n = 5) of the maximal reference value after the first intraarterial cisplatin infusion to 42.8% (range, 35.2% to 49.3%; n = 4) after the fifth infusion. Moreover, much like in the saline-receiving mice, mean values of superficial perfusion recorded 10 min after intervention were always lower than those before treatment. The perfusion in the front foot area was only minimally affected by repeated catheterization and cisplatin infusion. There was no decrease in perfusion after the first injection, and perfusion was only lowered to 90% (range, 77.5% to 100%; n = 4) after the fifth injection. Altogether, these results suggest that infusion of cisplatin into the femoral artery, unlike catheterization and saline infusion alone, caused some irreversible damage to the artery, resulting in progressively diminished perfusion around the ankle joint, with less marked effects in the front foot area of the operated leg. These observations prompted our detailed histologic analysis of the artery and surrounding tissue distal to the site of catheterization.
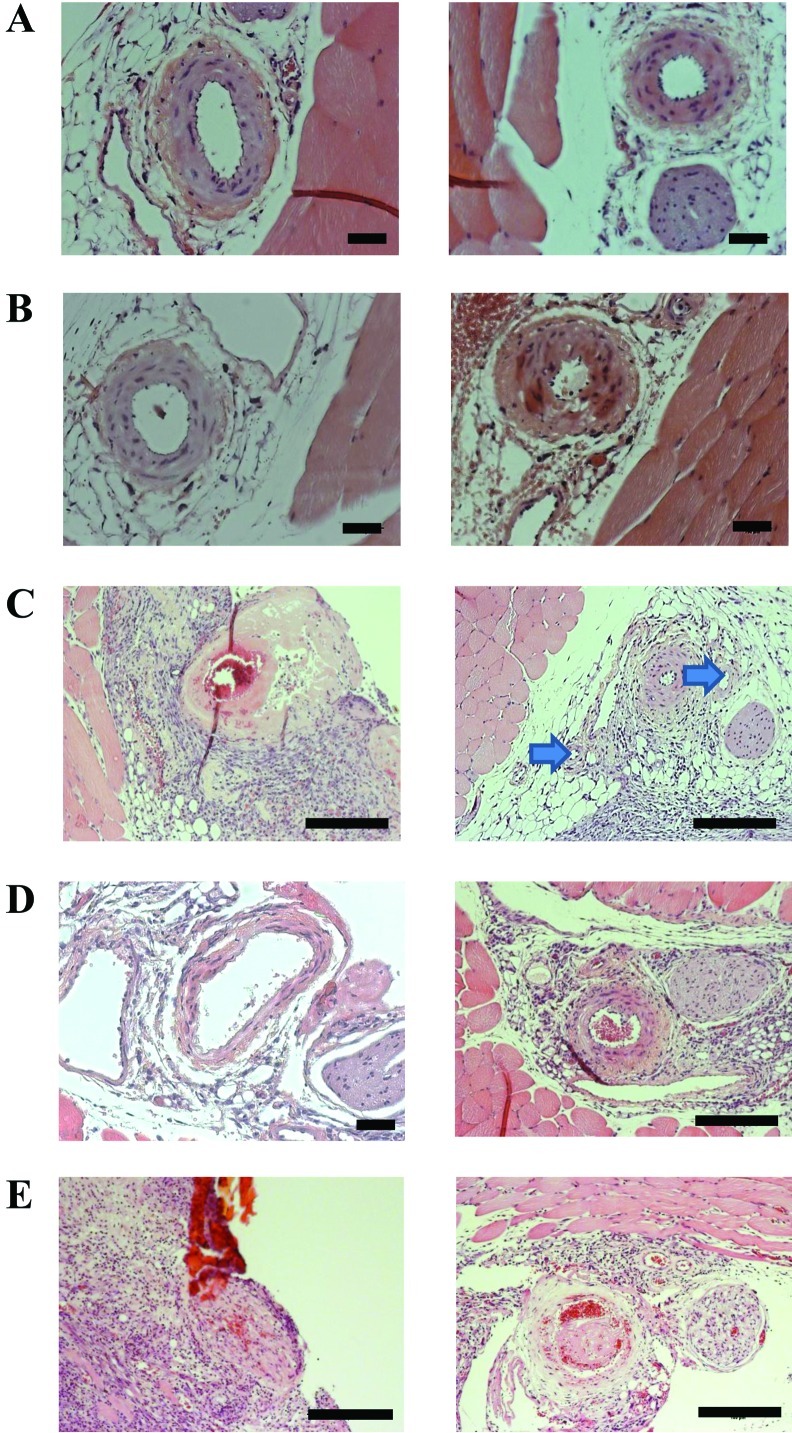
In this analysis, the morphology of operated legs was compared with that of nonoperated control legs by using cross-sections of formalin-fixed and paraffin-embedded tissue collected at intervals of 200 µm (10 sections per leg) for a distance of 3 to 4 mm around the catheterization site. Representative tissue sections collected from nonoperated legs of 2 randomly selected mice showed normal morphology of femoral arteries and surrounding tissue (Figure 4 A). Representative sections collected from legs that had undergone a single catheterization and saline infusion showed an intact arterial wall, with minimal swelling of the smooth muscle cells of the tunica media (Figure 4 B). Limb sections obtained after 3 interventions showed granulation tissue around the femoral artery, circumferential fibrinoid necrosis of the vessel wall, and incomplete occlusion of the lumen (Figure 4 C, left). One leg showed almost complete vessel occlusion, due to swelling of the endothelial layer, and collaterals (Figure 4 C, right). Limb tissue sections collected from operated legs of 3 mice that underwent 5 interventions showed partial fibrinoid necrosis of the media with a free lumen in 2 mice (Figure 4 D, left) and an almost normal morphology of the femoral artery in another mouse (Figure 4 D, right).
Figure 4.
Histology of nonoperated control and of catheterized and infused mouse hindlimbs. Images of 2 randomly selected (A) control legs; legs catheterized and infused with saline (B) once, (C) 3 times (arrows indicate collateral vessels), or (D) 5 times; and (E) a leg catheterized and infused with cisplatin 5 times (right panel) and from a leg after a failed fourth injection (left panel). Hematoxylin and eosin stain; scale bars, 100 μm.
In the group that received cisplatin, one mouse received only 3 successful catheterizations because the fourth preparation of the vessel failed. Histology of the operated leg of this particular animal 3 d after the last successful cisplatin treatment revealed that the remaining structures of the femoral artery had been displaced by surrounding granulation tissue, and a lumen was no longer visible (Figure 4 E, left). The analysis of limb tissue collected from the other 4 mice that were catheterized and treated with cisplatin 5 times showed medial necrosis but only partial vessel occlusion with fibrous thrombi (Figure 4 E, right), comparable to that of control mice. Taken together, the histologic analysis of tissue from catheterized legs collected near to and 3 to 4 mm distally to the site of catheterization of the femoral artery showed variable damage to the vessel depending primarily on the number of repeated catheterization. Most importantly, in 4 of 5 mice, catheterization and cisplatin infusion was successful in 5 consecutive interventions and, at the end of the series, the femoral arteries had a morphology of an at least partially functional vessel, consistent with the data from the perfusion measurements.
Discussion
IAC for osteosarcoma is somewhat controversial. A comparative multicenter clinical trial involving osteosarcoma patients revealed that efficacy did not differ between local intraarterial and systemic treatment with cisplatin, as reflected by the comparable percentages of high responders (greater than 90% tumor necrosis after chemotherapy).28 However, a recently published single-arm prospective study demonstrated that the 5-y survival rate of patients with localized osteosarcoma was considerably increased when the patients received individualized treatment that included combined intraarterial and intravenous neoadjuvant chemotherapy, surgery adjusted to tumor size, and arteriographically detected tumor neovascularization.11 The nonstandardized protocols for IAC in these 2 and other studies likely explain the discrepant findings. Therefore, the efficacy of intraarterial and systemic treatment of osteosarcoma with cisplatin, for example, needs to be investigated further in animal models under standardized conditions to obtain conclusive results. To this end, we designed the present study to establish a highly reproducible microsurgical method for repeated catheterization of the femoral artery for local cisplatin IAC of hindlimb primary tumors in experimental osteosarcoma mouse models.
The technique we report here is different from that described earlier, in which the jugular vein of mice was catheterized after approximately 50% of the circumference of the vessel was incised by using microscissors.15 We considered that such a large incision was too invasive for repeated catheterization of the mouse femoral artery. We therefore adapted a previous technique that mimics the Seldinger technique, thereby avoiding transverse incision of the vessel wall.10,24 For easy access, we separated the femoral artery from the surrounding tissue and placed a latex band underneath it. To insert a polyethylene catheter into the vessel, as recommended previously,10 we first punctured the femoral artery with a cone-shaped glass capillary, causing much less damage than that of an incision from microscissors. The main technical difficulty we encountered was placing the tip of the glass capillary in the middle of the anterior surface of the artery at a tangential angle of approximately 45° and applying appropriate pressure to perforate the anterior wall without damaging the posterior wall. This is a key step in the whole procedure and one that requires practice.
Our use of polyethylene catheters with an average outer diameter of 110 μm instead of 0.4 mm, as reported previously,10 we considered to be important for minimizing damage to the luminal surface of the vessel and subsequent scar formation and obliteration of the femoral artery after repeated catheterization. In this regard, necrosis of the media and complete loss of smooth muscle cells was reported to occur within 7 d after expansion of the carotid artery of rabbits with an oversized stent.6 The authors of the rabbit report postulated that expansion and compression of the vessel wall by the stent decreased the oxygenation of the media through compression of the vasa vasorum and, consequently, caused necrosis. In the current study, histologic analysis of arteries catheterized 3 times showed moderate medial necrosis around the injection site. However, it is important to note that, after the fifth intervention, the vessels appeared to have recovered partially from the intervention-associated vessel damage. This observation is consistent with those of previous authors, who reported remodeling of arteries after expansion-associated medial necrosis.6
Differences in tissue perfusion values determined with laser speckle contrast imaging in a specific ROI of the control leg of individual mice within a particular group at a particular time point were approximately 50%. We presume that these considerable differences in tissue perfusion between mice at the same ROI were due to differences in body temperature, the response to anesthesia, or blood loss during the interventions.
Perfusion measurements distal to the site of catheterization indicated a transient decrease in perfusion after saline infusion, likely due to dilatation of the vessel by the catheter that evoked vasoconstriction as reported.5 Full recovery within 3 d from this intervention-associated decrease in superficial perfusion was evident when the 5 preintervention blood flow measurements in individual saline-infused mice were compared. In the described surgical technique for catheterization of the mouse femoral artery, we avoided leakage through the injection site by using cone-shaped catheters that fit tightly against the vessel wall. In addition, slow manual infusion (350 µL over 2 min) of cisplatin further prevented local tissue-damaging contamination at the injection site. Small amounts of leaked infusion solution were removed by flushing the wound with 0.9% saline after surgery. Therefore, tissue damage due to remaining chemotherapeutic agent was not a problem. Nevertheless, the measurements of superficial perfusion before and after cisplatin infusions indicated a progressive nonreversible decrease in superficial perfusion around the ankle but not in the front foot of catheterized legs during the 5 interventions. The histologic analysis of infused arteries showed signs of irreversible tissue damage, but, importantly, the superficial perfusion measured after the fifth catheterization and infusion of cisplatin was only diminished by 50% relative to the values recorded before the first intervention. The vascular tissue-damaging effects of cisplatin causing cardiac ischemia, cerebrovascular insult and hypertension has been reported.7 We believe that the remaining blood supply in the leg is sufficient to maintain high local cisplatin concentrations, likely enhancing the efficacy of local compared with systemic cisplatin treatment in the intratibial osteosarcoma mouse model.
In conclusion, we established a reliable microsurgical method for repeated administration of chemotherapeutic agents through the femoral artery of mice with hindlimb experimental osteosarcoma. The key procedures of the method are the fabrication of polyethylene catheters with an optimal diameter of 110 ± 30 µm and the initial puncturing of the femoral arteries by using a glass capillary. Importantly, perfusion measurements by laser speckle contrast imaging reflected the histologic findings in catheterized arteries and can therefore be used for monitoring local tissue perfusion during intraarterial drug administration. The present study sets the stage for future investigations that compare the efficacies of systemic and IAC treatment of intratibial metastasizing osteosarcoma under standardized conditions.
Acknowledgments
We thank Dr Vera Maria Sallen for technical support during this work. This study was supported by a grant from the Denver Clinic for Extremities at Risk (Presbyterian–St Luke's Medical Center, Denver CO), by the Swiss Association Balgrist and the Balgrist Foundation, the Walter L and Johanna Wolf Foundation (Zurich, Switzerland), the Swiss National Science Foundation (SNF), and the University of Zurich.
References
- 1.Alkureishi LW, De Bree R, Ross GL. 2006. RadPlat: an alternative to surgery? Oncologist 11:469–480 [DOI] [PubMed] [Google Scholar]
- 2.Bacci G, Briccoli A, Ferrari S, Longhi A, Mercuri M, Capanna R, Donati D, Lari S, Forni C, Depaolis M. 2001. Neoadjuvant chemotherapy for osteosarcoma of the extremity: long-term results of Rizzoli's 4th protocol. Eur J Cancer 37:2030–2039 [DOI] [PubMed] [Google Scholar]
- 3.Bertino JR, Boston B, Capizzi RL. 1975. The role of chemotherapy in the management of cancer of the head and neck: a review. Cancer 36:752–758 [DOI] [PubMed] [Google Scholar]
- 4.Collins JM. 1984. Pharmacologic rationale for regional drug delivery. J Clin Oncol 2:498–504 [DOI] [PubMed] [Google Scholar]
- 5.Davis MJ, Donovitz JA, Hood JD. 1992. Stretch-activated single-channel and whole-cell currents in vascular smooth muscle cells. Am J Physiol 262:C1083–C1088 [DOI] [PubMed] [Google Scholar]
- 6.Dirsch O, Dahmen U, Fan LM, Gu YL, Shen K, Wieneke H, Erbel R. 2004. Media remodeling—the result of stent-induced media necrosis and repair. Vasa 33:125–129 [DOI] [PubMed] [Google Scholar]
- 7.Doll DC, Ringenberg QS, Yarbro JW. 1986. Vascular toxicity associated with antineoplastic agents. J Clin Oncol 4:1405–1417 [DOI] [PubMed] [Google Scholar]
- 8.Fiorentini G, Tsetis D, Bernardeschi P, Varveris C, Rossi S, Kalogeraki A, Athanasakis E, Dentico P, Kanellos P, Biancalani M, Almarashdah S, Zacharioudakis G, Saridaki Z, Chalkiadakis G, Xynos E, Zoras O. 2003. First-line intraarterial chemotherapy (IAC) with epirubicin and mitoxantrone in locally advanced breast cancer. Anticancer Res 23:4339–4345 [PubMed] [Google Scholar]
- 9.Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, Graf N, Heise U, Jurgens H, Kotz R, Salzer-Kuntschik M, Weinel P, Werner M, Winkler K. 1998. Long-term results of the cooperative German–Austrian–Swiss osteosarcoma study group's protocol CosteosarcomaS86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol 9:893–899 [DOI] [PubMed] [Google Scholar]
- 10.Fukui S, Nawshiro H, Wada K, Shima K, Hallenbeck JM. 2001. A new method to catheterize a femoral artery in mice using a nylon suture as a ‘guide wire.’ Neurol Res 23:655–656 [DOI] [PubMed] [Google Scholar]
- 11.Hugate RR, Wilkins RM, Kelly CM, Madsen W, Hinshaw I, Camozzi AB. 2008. Intraarterial chemotherapy for extremity osteosarcoma and MFH in adults. Clin Orthop Relat Res 466: 1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klopp CT, Alford TC, Bateman J, Berry GN, Winship T. 1950. Fractionated intraarterial cancer: chemotherapy with methyl bis amine hydrochloride—a preliminary report. Ann Surg 132:811–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarter DH, Doughty JC, Cooke TG, Mcardle CS, Reid AW. 1998. Selective angiographically delivered regional chemotherapy in patients with locally advanced or recurrent breast cancer: a feasibility study. J Vasc Interv Radiol 9:91–96 [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra R, Ibrahim R, Eckardt A, Driemel O, Singh M. 2011. Novel strategies in therapy of head and neck cancer. Curr Cancer Drug Targets 11:465–478 [DOI] [PubMed] [Google Scholar]
- 15.Mokhtarian A, Meile MJ, Even PC. 1993. Chronic vascular catheterization in the mouse. Physiol Behav 54:895–898 [DOI] [PubMed] [Google Scholar]
- 16.Nicolaidis S, Rowland N, Meile MJ, Marfaing-Jallat P, Pesez A. 1974. A flexible technique for long-term infusions in unrestrained rats. Pharmacol Biochem Behav 2:131–136 [DOI] [PubMed] [Google Scholar]
- 17.Pacetti P, Mambrini A, Paolucci R, Sanguinetti F, Palmieri B, Della Seta R, Muttini MP, Fiorentini G, Cantore M. 2006. Intraarterial chemotherapy: a safe treatment for elderly patients with locally advanced breast cancer. In Vivo 20:761–764 [PubMed] [Google Scholar]
- 18. Parlaveccio G. 1899. Sul lavaggio antisettico interstizile dei tessut dolla vie arteriosa. Policlinico 6: 667–674.
- 19.Qureshi AI, Suri MF, Khan J, Sharma M, Olson K, Guterman LR, Hopkins LN. 2001. Superselective intraarterial carboplatin for treatment of intracranial neoplasms: experience in 100 procedures. J Neurooncol 51:151–158 [DOI] [PubMed] [Google Scholar]
- 20.Robbins KT, Kumar P, Wong FS, Hartsell WF, Flick P, Palmer R, Weir AB, 3rd, Neill HB, Murry T, Ferguson R, Hanchett C, Vieira F, Bush A, Howell SB. 2000. Targeted chemoradiation for advanced head and neck cancer: analysis of 213 patients. Head Neck 22:687–693 [DOI] [PubMed] [Google Scholar]
- 21.Robbins KT, Storniolo AM, Kerber C, Seagren S, Berson A, Howell SB. 1992. Rapid superselective high-dose cisplatin infusion for advanced head and neck malignancies. Head Neck 14:364–371 [DOI] [PubMed] [Google Scholar]
- 22.Sato T. 2010. Locoregional management of hepatic metastasis from primary uveal melanoma. Semin Oncol 37:127–138 [DOI] [PubMed] [Google Scholar]
- 23.Schouwenburg PF, Van Putten LM, Snow GB. 1980. External carotid artery infusion with single-and multiple-drug regimens in the rat. Cancer 45:2258–2264 [DOI] [PubMed] [Google Scholar]
- 24. Seldinger SI. 1953. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta Radiol 39: 368–376. [DOI] [PubMed]
- 25.Shields CL, Shields JA. 2010. Intra-arterial chemotherapy for retinoblastoma: the beginning of a long journey. Clin Experiment Ophthalmol 38:638–643 [DOI] [PubMed] [Google Scholar]
- 26.Stewart DJ, Benjamin RS, Zimmerman S, Caprioli RM, Wallace S, Chuang V, Calvo D, 3rd, Samuels M, Bonura J, Loo TL. 1983. Clinical pharmacology of intraarterial cis-diamminedichloroplatinum(II). Cancer Res 43:917–920 [PubMed] [Google Scholar]
- 27.Wilkins RM, Cullen JW, Camozzi AB, Jamroz BA, Odom L. 2005. Improved survival in primary nonmetastatic pediatric osteosarcoma of the extremity. Clin Orthop Relat Res 438:128–136 [DOI] [PubMed] [Google Scholar]
- 28.Winkler K, Bielack S, Delling G, Salzer-Kuntschik M, Kotz R, Greenshaw C, Jurgens H, Ritter J, Kusnierz-Glaz C, Erttmann R, Gädicke G, Graf N, Ladenstein R, Leyvraz S, Mertens R, Weinel P. 1990. Effect of intraarterial versus intravenous cisplatin in addition to systemic doxorubicin, high-dose methotrexate, and ifosfamide on histologic tumor response in osteosarcoma (study CosteosarcomaS86). Cancer 66:1703–1710 [DOI] [PubMed] [Google Scholar]