Abstract
Rationale
Alterations in cost–benefit decision making accompany numerous neuropsychiatric conditions, including schizophrenia, attention deficit hyperactivity disorder, and addiction. Central cholinergic systems have been linked to the etiology and/or treatment of many of these conditions, but little is known about the role of cholinergic signaling in cost–benefit decision making.
Objectives
The goal of these experiments was to determine how cholinergic signaling is involved in cost–benefit decision making, using a behavioral pharmacological approach.
Methods
Male Long-Evans rats were trained in either “probability discounting” or “delay discounting” tasks, in which rats made discrete-trial choices between a small food reward and a large food reward associated with either varying probabilities of omission or varying delays to delivery, respectively. The effects of acute administration of different doses of nicotinic and muscarinic acetylcholine receptor agonists and antagonists were assessed in each task.
Results
In the probability discounting task, acute nicotine administration (1.0 mg/kg) significantly increased choice of the large risky reward, and control experiments suggested that this was due to robust nicotine-induced impairments in behavioral flexibility. In the delay discounting task, the muscarinic antagonists scopolamine (0.03, 0.1, and 0.3 mg/kg) and atropine (0.3 mg/kg) both significantly increased choice of the small immediate reward. Neither mecamylamine nor oxotremorine produced reliable effects on either of the decision making tasks.
Conclusions
These data suggest that cholinergic receptors play multiple roles in decision making contexts which include consideration of reward delay or probability. These roles should be considered when targeting these receptors for therapeutic purposes.
Keywords: Cholinergic, Nicotinic, Muscarinic, Delay discounting, Probability discounting, Impulsivity, Risk, Perseveration
Introduction
Cost–benefit decision making is characterized by the ability to make choices among reward options which differ in both reward magnitudes and costs, the latter of which can include such variables as delay to reward delivery and probability of reward omission. Optimal cost–benefit decision making yields maximum rewards with minimum costs, whereas poor cost–benefit decision making (termed “impulsive” in the case of suboptimal delay-based decision making and “risky” in the case of suboptimal probability-based decision making) results in larger costs and/or smaller rewards. In humans, such suboptimal decision making can have a large impact on health, finances, social relationships, and overall quality of life.
Suboptimal cost–benefit decision making is a prominent feature of numerous neuropsychiatric conditions, including schizophrenia, Alzheimer’s disease, attention deficit hyper-activity disorder (ADHD), and addiction (Gleichgerrcht et al. 2010; Kalivas and Volkow 2005; Thompson et al. 2007; Weiler et al. 2009). These conditions are linked in that central cholinergic systems have been implicated in their etiology and/or treatment (Mukhin et al. 2008; Potter and Newhouse 2005; Warpman and Nordberg 1995; Yip et al. 2009). Such links suggest that central cholinergic systems may play a role in the altered cost–benefit decision making observed in these conditions; however, in contrast to other neurotransmitter systems (e.g., dopamine), there is surprisingly little known about the contributions of cholinergic signaling to cost–benefit decision making.
Discounting tasks are commonly used across species to assess cost–benefit decision making. Such tasks generally involve presenting subjects with the opportunity to repeatedly choose between two reward options: a large reward that is associated with varying costs (e.g., delay to delivery) that devalue this reward and a small reward with few or no costs (Mazur 1987). One advantage of such tasks is that they allow analysis of choice proportion gradients, as subjects will shift their choice toward or away from the large reward, depending on its cost. In addition, they can offer dissociable information about how changes in degree of preference relate to changes in reward magnitude, cost size, and overall reward values (Ho et al. 1999; Killeen 2011). Notably, there is some evidence that the mechanisms underlying the effects of different types of reward costs on behavior (e.g., delay vs. probability) are dissociable, both at the behavioral and neural levels (Floresco et al. 2008; Rachlin et al. 1991; Simon et al. 2009).
Both acute and chronic administration of nicotine in rats cause increases in choice of small immediate vs. large delayed rewards (i.e., greater impulsive choice) in tests of delay discounting (Dallery and Locey 2005; Kolokotroni et al. 2011). These findings concur with those from studies of human smokers, who, like nicotine-exposed rats, also show greater impulsive choice relative to nonsmokers and ex-smokers (Bickel et al. 1999; Field et al. 2006), although acute nicotine in nonsmoking adults with ADHD may actually decrease impulsive choice (Potter and Newhouse 2008). Psychopharmacological data in delay discounting tasks using cholinergic drugs other than nicotine are limited; however, acute administration of mecamylamine (a nonselective nicotinic antagonist) to adults with ADHD is reported to increase impulsive choice (Potter et al. 2009), while acute mecamylamine administration in rats has no effect (Kolokotroni et al. 2011; see also Xie et al. 2012).
Data on cholinergic contributions to probability discounting are even more limited. Such decision making is reported to be altered in smokers compared to nonsmokers, although the direction of these alterations (increased or decreased risky decision making) is unclear (Mitchell 1999; Reynolds et al. 2004). In addition, recent data from our laboratory show that acute nicotine administration in rats decreases preference for large rewards associated with a risk of punishment vs. small safe rewards (i.e., decreased risky choice; Mitchell et al. 2011). To gain a broader understanding of the role of cholinergic systems in cost–benefit decision making, we examined the effects of acute administration of drugs acting at nicotinic and muscarinic cholinergic receptors on delay and probability discounting. It was expected that drugs acting at nicotinic receptors would influence performance on both tasks, although given the mixed findings in the literature, the expected direction of any such effects was unclear. To our knowledge, there are no prior studies of the role of muscarinic receptors in decision making.
Materials and methods
Subjects
The subjects were male Long-Evans rats weighing 250–275 g upon arrival (Charles River Laboratories, Wilmington, NC, USA). Rats were housed individually in a climate-controlled vivarium (25 °C) at either Texas A&M University or University of Florida. Four groups of rats were used for these experiments, which were conducted in the following order: the first group (n=15) was tested in experiments 1.1–1.4; the second group (n=16) in experiments 2.1, 2.2, 2.4, and 1.6; the third group (n=16) in experiments 2.3 and 2.5; and the fourth group (n=16) in experiment 1.5. Rats had unrestricted access to food and water, except as noted below. Testing took place during the light cycle of a 12-h light/dark schedule (lights on 0800–2000) and was conducted according to the “Guide for the Care and Use of Laboratory Animals” (National Academy of Sciences, Washington, DC, USA) and met all National Institutes of Health and institutional animal care guidelines. Rats acclimated to vivarium conditions for at least 1 week before the experiments began. Prior to testing, rats were food restricted to 85 % of their free feeding weight over 5 days. To maintain them at this weight, they were limited to 15 g of food/day (in addition to food earned during testing), with allowances for growth.
Behavioral apparatus
All testing took place in eight identical standard rat behavioral test chambers (Coulbourn Instruments, Whitehall, PA, USA; for details, see Mendez et al. 2010). A recessed food trough into which grain-based food pellet rewards (PJAI, Test Diet, 45 mg) were delivered was equipped with a photobeam to detect head entries and was placed in the center of the front wall of the chambers. Retractable response levers were located on each side of the food trough, and each chamber was equipped with an overhead infrared activity monitor. The chambers were interfaced with a computer running Graphic State 3.01 software (Coulbourn Instruments) to control stimulus deliveries and record data.
Behavioral protocols
Probability discounting task
Detailed shaping and testing procedures have been described previously (Mendez et al. 2010). Briefly, each 60-min session in the probability discounting task consisted of five blocks of 18 trials each. On each trial, a nose poke into the food trough extinguished the food trough light and triggered extension of either a single lever (forced choice trials) or both levers simultaneously (free choice trials). A press on one lever (either left or right, balanced across rats) resulted in the delivery of a single food pellet (the small reward). A press on the other lever resulted in the delivery of four food pellets (the large reward). Failures to press either lever were scored as omissions. During the first block of trials, the probability of large reward delivery was 100 %. During each of the next four blocks, the probability of large reward delivery decreased (50, 25, 12.5, and 0 %, respectively). Each 18-trial block began with eight forced choice trials (four for each lever) used to expose the rats to the reward probabilities in effect for that block, followed by ten free choice trials. Rats were tested until stable performance was achieved, at which point drug tests were initiated as described below.
Perseveration tests
To determine whether the effects of nicotine in the probability discounting task in experiment 1.2 were due to alterations in behavioral flexibility, rats were tested in several variants of the probability discounting task. In the first of these, rats were tested in a probability discounting task with ascending probabilities of large reward delivery across trial blocks (0, 12.5, 25, 50, and 100 %). Rats were then tested in a “within-session shift” task, in which delivery of the large reward switched between blocks 2 and 3 from guaranteed to omitted entirely (100, 100, 0, 0, and 0 %).
Delay discounting task
Detailed procedures for training and testing in this task have been described previously (Evenden and Ryan 1996; Mitchell et al. 2012). Briefly, each 60-min session consisted of five blocks of 12 trials each. A nose poke into the food trough during these trials extinguished the food trough light and triggered the extension of either a single lever (forced choice trials) or both levers simultaneously (free choice trials). Each block consisted of two forced choice trials followed by ten free choice trials. A press on one lever (either left or right, balanced across rats) resulted in one food pellet delivered immediately. A press on the other lever resulted in three food pellets delivered after a variable delay, the duration of which increased across blocks (0, 4, 8, 16, and 32 s). Drug tests were initiated once stable performance was achieved.
Drug tests
Five different cholinergic drugs were administered acutely prior to testing (nicotine hydrogen tartrate (measured as the weight of the salt), mecamylamine hydrochloride, oxotremorine M, scopolamine hydrobromide trihydrate, and atropine; for doses and timing of injections, see Table 1; Locey and Dallery 2009; O’Hare et al. 1997). Administration of vehicle and three doses of each drug occurred over a period of 8 days using a within-subjects design according to the following schedule: dose 1, no injections; dose 2, no injections; dose 3, no injections; dose 4, no injections (with the exception of scopolamine, for which 3 days elapsed between doses). For the perseveration tests (experiment 1.6), only vehicle and the highest dose of nicotine were used. The order in which the doses of each drug (including vehicle) were administered was counterbalanced across rats. Drugs were purchased from Sigma and administered in 0.9 % saline vehicle at a volume of 1 ml/kg. Between tests with different drugs, rats received 12–33 sessions in their respective tasks to reestablish stable performance.
Table 1.
Cholinergic drug timing and doses
| Dose (mg/kg) | Nicotine | Mecamylamine | Oxotremorine | Scopolamine | Atropinea |
|---|---|---|---|---|---|
| Time of injection before testing (min) | 10 | 10 | 15 | 0 | 15 |
| Vehicle | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Low | 0.1 | 0.5 | 0.01 | 0.03 | 0.3/0.03 |
| Medium | 0.3 | 1.0 | 0.03 | 0.1 | 1.0/0.1 |
| High | 1.0 | 2.0 | 0.1 | 0.3 | 3.0/0.3 |
Doses for experiment 1.5/2.5
Statistical analysis
Raw data files were exported from Graphic State and compiled using a custom macro written for Microsoft Excel (Dr. Jonathan Lifshitz, University of Kentucky, Lexington, KY, USA). Statistical analyses were conducted in SPSS 16.0. Response omissions and intertrial interval locomotor activity on drug testing days were assessed using one-way repeated-measures analysis of variance (ANOVA) with Tukey’s honestly significant difference (HSD) post hoc tests when appropriate. The main dependent variable of interest was percent choice of the large reward (number of choice trials in which the large reward was chosen divided by the total number of choice trials completed). Rats that omitted seven or more of the ten choice trials in any block were excluded from the analysis for that experiment. Stable behavior was determined using two-way repeated-measures ANOVA (session×probability/delay) and was defined by the absence of a main effect or interaction involving session (Cardinal et al. 2000). Rats required 25–45 sessions to achieve stable behavior prior to drug testing. Analyses of choice behavior on drug testing days were conducted using two-way repeated-measures ANOVA (drug dose×probability/delay) with Tukey’s HSD post hoc tests when warranted. In all cases, p values <0.05 were considered significant.
Results
Experiment 1: probability discounting
In all experiments, the main effect of trial block (probability) was significant (Fs>21.56, ps<0.001) and will not be reported further.
Experiment 1.1: nicotine
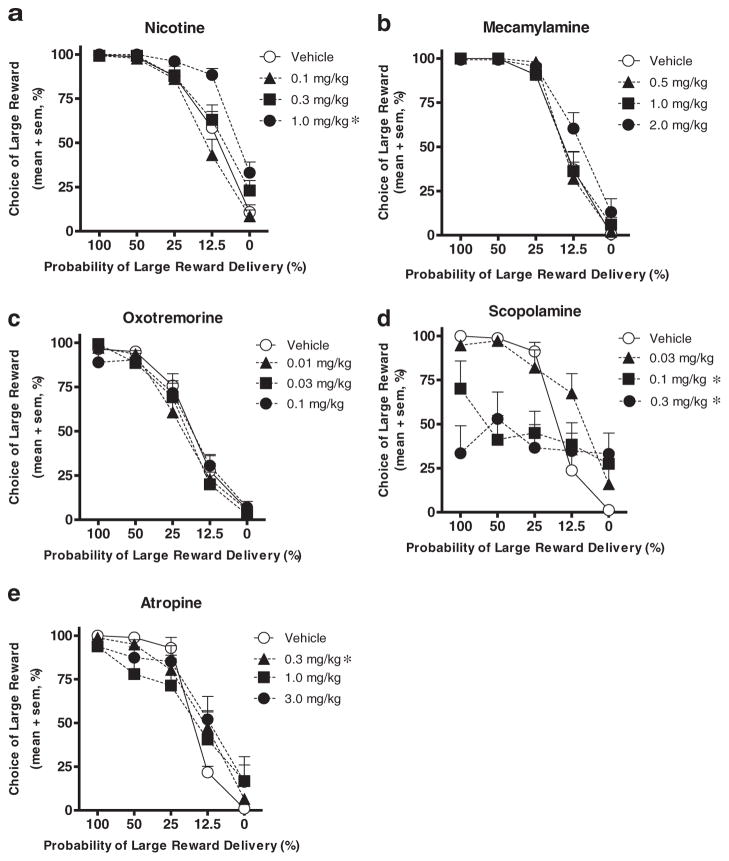
Nicotine produced a significant main effect of dose (F(3,36)= 13.04, p<0.001) as well as a significant interaction between dose and block (F(12,144)=4.64, p <0.001). Post hoc analyses of the dose×block interaction showed significant effects of nicotine on choice in blocks 4 and 5 (ps<0.01). Individual post hoc comparisons also revealed that the highest dose significantly increased choice of the large risky reward compared to vehicle (p<0.01) (Fig. 1a). There were also main effects of nicotine on both trial omissions (F(3,42) =6.83, p<0.01) and locomotor activity (F(3,30) =7.16, p<0.01; Table 2). Post hoc analyses revealed that the high dose increased trial omissions, whereas the medium dose increased locomotor activity (ps <0.05).
Fig. 1.
Choice performance in the probability discounting task following administration of low (black triangles), middle (black squares), and high (black circles) doses of cholinergic drugs was compared to performance following vehicle (white circles). Nicotine (a) increased while scopolamine (d) decreased mean percent choice of the large reward. Atropine (e) decreased choice of the large reward at high probabilities but increased choice at low probabilities. Neither mecamylamine (b) nor oxotremorine (c) significantly affected choice performance. Error bars indicate the standard error of the mean (SEM). *p<0.05 compared to vehicle
Table 2.
Effects of cholinergic drugs on response omissions and locomotor activity during testing in the probability discounting task
| Dose | Nicotine | Mecamylamine | Oxotremorine | Scopolamine | Atropine | |
|---|---|---|---|---|---|---|
| 1.1 | 1.2 | 1.3 | 1.4 | 1.5 | ||
| Mean percentage of choice trials omitted | Vehicle | 0.3 | 2.0 | 2.0 | 4.8 | 4.5 |
| Low | 0.1 | 1.2 | 1.5 | 26.0* | 22.4* | |
| Medium | 0.0 | 1.6 | 1.0 | 52.8* | 41.6* | |
| High | 6.1* | 2.1 | 4.8 | 53.2* | 49.3* | |
| Mean ITI locomotor activity | Vehicle | 2,382 | 1,618 | 1,983 | 2,164 | 1,395 |
| Low | 2,621 | 1,463 | 1,984 | 1,560* | 1,383 | |
| Medium | 2,939* | 1,637 | 1,784 | 1,200* | 1,230 | |
| High | 1,949 | 1,490 | 1,458* | 1,496* | 1,319 |
p<0.05, significantly different from vehicle
Experiment 1.2: mecamylamine
Mecamylamine produced neither a main effect of dose nor interaction between dose and block, although both effects trended toward increased choice of the large risky reward (Fig. 1b). Mecamylamine also had no effects on trial omissions or locomotor activity (Table 2).
Experiment 1.3: oxotremorine
Oxotremorine produced neither a main effect of dose nor interaction between dose and block (Fig. 1c). Oxotremorine also had no effect on trial omissions, but did affect locomotor activity (F(3,39)=5.6, p<0.01; Table 2), with the highest dose causing a significant reduction (p<0.01).
Experiment 1.4: scopolamine
Scopolamine produced a significant main effect of dose (F(3,28) =7.53, p <0.01) as well as an interaction between dose and block (F(12,84) =5.52, p <0.01). Post hoc analyses of the dose×block interaction showed a significant effect of scopolamine on choice in blocks 1, 2, 3, and 4 (ps<0.05). Post hoc comparisons between each dose and vehicle conditions also revealed that the medium and high doses significantly decreased choice of the large risky reward (ps <0.05) (Fig. 1d). Moreover, scopolamine had significant effects on both response omissions (F(3,42) =28.49, p<0.01) and locomotor activity (F(3,33) =9.49, p<0.01; Table 2), with all doses increasing omissions and decreasing locomotor activity compared to vehicle (ps<0.01).
Experiment 1.5: atropine
Because scopolamine produced considerable disruptions in task performance, a second muscarinic antagonist was used to examine the effects of muscarinic blockade on probability discounting. Acute administration of the middle and high doses of atropine greatly increased response omissions (ten and nine rats omitted more than seven of the ten choice trials in at least one block with the medium and high dose, respectively), resulting in insufficient data for reliable statistical analyses. Analysis of data from vehicle and the low dose of atropine alone revealed no significant main effect of dose, but a significant interaction between dose and block (F(4,36)=7.68, p<0.001), such that choice of the large reward was reduced at high probabilities and increased at low probabilities of delivery. Post hoc analyses of the dose×block interaction showed a significant effect of atropine on choice in blocks 4 and 5 (ps<0.05). Although the middle and high doses could not be analyzed statistically, data from the rats that were not omitted are plotted in Fig. 1e and suggest a pattern of choice performance comparable to that with the low dose. Atropine also had significant effects on response omissions (F(3,45) =17.55, p<0.01; Table 2), with all doses increasing omissions compared to vehicle (ps <0.01). There was no effect of atropine on locomotor activity.
Experiment 1.6: effects of nicotine on perseverative choice behavior
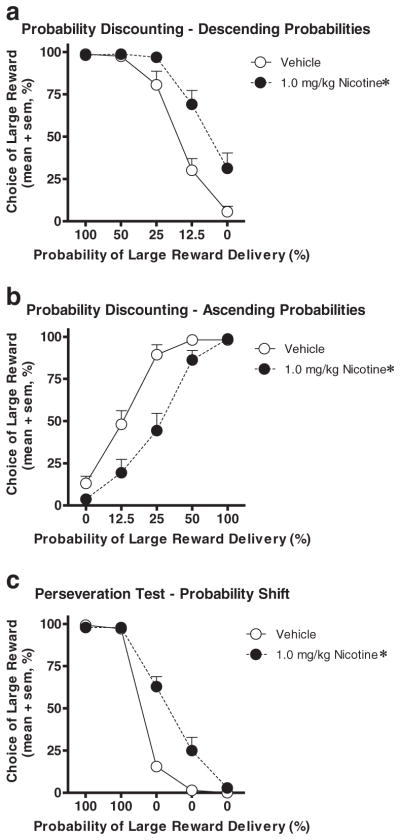
The pattern of effects of nicotine on the probability discounting task in experiment 1.2 (increased choice of the large risky reward) could be interpreted as an increase in perseverative responding (i.e., persistent choice of a reward option even after it becomes disadvantageous) rather than an increase in preference for large risky rewards per se (St. Onge et al. 2010). To address this possibility, the most effective dose of nicotine from experiment 1.2 was tested on several variants of the probability discounting task. Prior to each of these tests, rats achieved stable performance on the task variant being tested. The first of these tests was a replication of experiment 1.2, which again showed that acute nicotine (1.0 mg/kg) increased choice of the large risky reward (main effect of nicotine, F(1,15)=19.83, p<0.05; interaction between drug condition and block, F(4,60)=5.74, p<0.01; Fig. 2a). Post hoc analyses of the dose×block interaction showed a significant effect of nicotine on choice in blocks 4 and 5 (ps<0.01). In the second test, the order of probabilities of reward delivery was reversed (beginning with 0 % and proceeding up to 100 %). Because this task variant involved the same risks of reward omission as the “regular” version of the task, but presented them in the opposite order, it allowed a dissociation between the two possible interpretations of the effects in experiment 1.2 (increased risk-taking vs. increased perseveration) (St. Onge and Floresco 2009). Again, there was both a main effect of drug condition (F(1,15) =11.07, p <0.01) and an interaction between drug condition and block (F(4,60)=5.49, p<0.01); however, in this version of the task, nicotine decreased choice of the large risky reward relative to vehicle (Fig. 2b). Post hoc analyses of the dose×block interaction in this test showed a significant effect of nicotine on choice in blocks 2 and 3 (ps<0.05). In the third test, the large reward was delivered with 100 % probability for the first two blocks and 0 % probability for the final three blocks. This test eliminated the need for calculations of reward probabilities and was thus a more “pure” test of perseverative behavior. As in the first two tests, there was both a main effect of drug (F(1,13)=23.75, p<0.01) and an interaction between drug and block (F(4,52)=22.13, p<0.01), with nicotine causing elevated choice of the large reward across multiple blocks of 0 % probability of delivery (Fig. 2c). Additionally, post hoc analyses of the dose×block interaction showed a significant effect of nicotine on choice in blocks 3 and 4 (ps<0.05).
Fig. 2.
Choice performance in the probability discounting task following administration of a high dose of nicotine (black circles) was compared to performance following vehicle (white circles). Nicotine increased choice of the large reward in versions of the task with descending (a) and shifting (c) large reward probabilities, but decreased choice of the large reward in a version of the task with ascending large reward probabilities (b). Error bars indicate the SEM. *p<0.05 compared to vehicle
Experiment 2: delay discounting
In all experiments, the main effect of trial block (delay) was significant (Fs>14.58, ps<0.001) and will not be reported further.
Experiment 2.1: nicotine
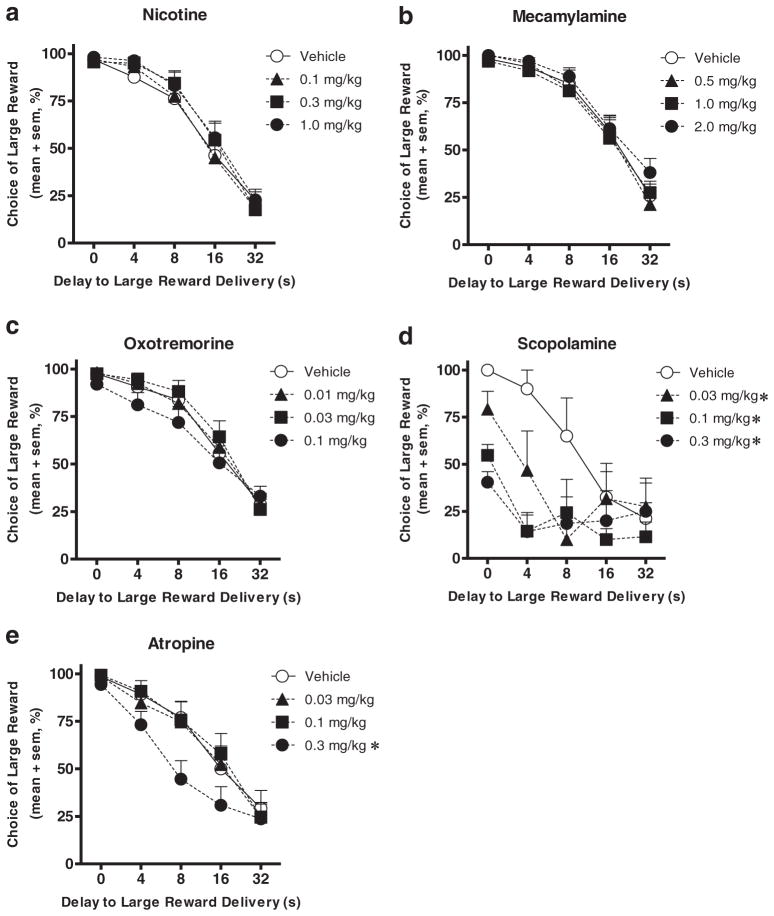
Nicotine produced neither a main effect of dose nor an interaction between dose and block (Fig. 3a). There was also no effect on response omissions, although there was an effect on locomotor activity (F(3,45) =2.91, p<0.05), with both the middle and high doses of nicotine producing a significant increase relative to vehicle (ps<0.05; Table 3).
Fig. 3.
Choice performance in the delay discounting task following administration of low (black triangles), middle (black squares), and high (black circles) doses of cholinergic drugs was compared to performance following vehicle (white circles). Both scopolamine (d) and atropine (e) decreased choice of the large reward, whereas nicotine (a), mecamylamine (b), and oxotremorine (c) did not have any significant effects on choice performance. Error bars indicate the SEM. *p<0.05 compared to vehicle
Table 3.
Effects of cholinergic drugs on response omissions and locomotor activity during testing in the delay discounting task
| Dose | Nicotine | Mecamylamine | Oxotremorine | Scopolamine | Atropine | |
|---|---|---|---|---|---|---|
| 2.1 | 2.2 | 2.3 | 2.4 | 2.5 | ||
| Mean percentage of choice trials omitted | Vehicle | 0.1 | 0.5 | 0.1 | 1.5 | 3.2 |
| Low | 0.3 | 0.0 | 0.4 | 45.6* | 3.3 | |
| Medium | 0.1 | 0.0 | 0.3 | 59.4* | 3.2 | |
| High | 0.1 | 0.3 | 0.5 | 66.3* | 7.2* | |
| Mean ITI locomotor activity | Vehicle | 2,883 | 2,277 | 3,136 | 3,170 | 2,372 |
| Low | 3,006 | 2,325 | 3,175 | 2,816 | 2,348 | |
| Medium | 3,729* | 2,219 | 3,278 | 2,769 | 2,311 | |
| High | 3,780* | 2,209 | 2,748* | 3,354 | 1,815* |
p<0.05, significantly different from vehicle
Experiment 2.2: mecamylamine
Mecamylamine produced no effects on choice behavior, although there was a trend toward increased choice of the large reward (decreased impulsive choice; Fig. 3b). There were also no effects on response omissions or locomotor activity.
Experiment 2.3: oxotremorine
Oxotremorine produced neither a main effect of dose nor an interaction between dose and block, although there was a trend toward decreased choice of the large delayed reward (increased impulsive choice; Fig. 3c). There was also no effect on response omissions, although there was an effect on locomotor activity (F(3,45)=4.07, p<0.05), with the high dose producing a near-significant decrease relative to vehicle (Table 3).
Experiment 2.4: scopolamine
Scopolamine produced a significant main effect of dose (F(3,12) =12.06, p <0.01), as well as a significant interaction between dose and block (F(12,36) =2.96, p <0.01). Post hoc analyses of the dose×block interaction showed a significant effect of scopolamine on choice in blocks 1 and 2 (ps<0.01). Post hoc analyses also revealed that all doses of scopolamine significantly reduced choice of the large delayed reward (Fig. 3d). There was also a main effect on response omissions (F(3,42) =29.70, p<0.01), with all doses significantly increasing the number of omitted trials (ps<0.01), but no effect on locomotor activity (Table 3).
Experiment 2.5: atropine
Atropine produced a significant main effect of dose (F(3,36) =6.81, p<0.01) as well as a significant interaction between dose and block (F(12,144)=2.74, p<0.01). Post hoc analyses of the dose×block interaction showed a significant effect of atropine on choice in blocks 2 and 3 (ps<0.01). Post hoc analyses also revealed that the high dose significantly decreased choice of the large delayed reward (increased impulsive choice, p<0.05; Fig. 3e). There were also significant main effects of atropine on trial omissions (F(3,45) =8.89, p <0.01) and locomotor activity (F(3,45) =4.03, p<0.05), with the high dose both increasing trial omissions and decreasing locomotor activity relative to vehicle conditions (ps<0.01; Table 3).
Locomotor activity
There was considerable variation in locomotor activity under vehicle conditions across experiments. Some of this variation may have been due to the different cohorts of rats used for different experiments; however, note that drug effects on locomotor activity were similar across experiments 1 and 2 (see Tables 2 and 3), suggesting that such variation did not account for the magnitude or direction of these effects.
Discussion
The contributions of cholinergic systems to cost–benefit decision making are poorly understood, despite the fact that cholinergic signaling plays an important role in the etiology and/or treatment of many neuropsychiatric disorders in which altered decision making is prominent (Mukhin et al. 2008; Potter and Newhouse 2005; Warpman and Nordberg 1995; Yip et al. 2009). The overall goal of these experiments was to characterize the roles of cholinergic receptors in two forms of cost–benefit decision making, using a behavioral pharmacological approach. The results suggest both common and unique roles for the two major types of cholinergic receptors (nicotinic and muscarinic) in probability and delay discounting.
Nicotinic receptors and cost–benefit decision making
Acute activation of nicotinic receptors via the nonselective agonist nicotine produced a robust increase in choice of the large “risky” reward in the probability discounting task. However, in a modified version of the probability discounting task in which the order of probabilities of reward omission decreased (rather than increased) across the test session, nicotine decreased choice of the large risky reward. This finding suggests that the effects of nicotine on choice behavior in the “standard” version of the task were not due to increases in risk-taking per se, but instead reflected impaired behavioral flexibility (i.e., perseveration), such that nicotine caused persistent choice of the option selected in early blocks of trials, even after it became disadvantageous in later blocks. This persistence was also evident in another modified version of the task, in which the probabilities of reward omission were either 0 or 100 %. In this version, which eliminated the need to detect and respond to changes in reward probability, nicotine also caused persistent choice of the disadvantageous option, providing further evidence for nicotine-induced perseveration. Interestingly, effects similar to those observed here with nicotine were found following acute administration of amphetamine in the probability discounting task (St. Onge and Floresco 2008; St. Onge et al. 2010). Like nicotine, amphetamine also increased choice of the large risky reward when the probability of reward omission increased over the course of the test session, but decreased choice of the large risky reward when the probability of omission decreased. This pattern of behavior was interpreted as an inability to modify the “perceived relative value” of the initially chosen reward on the basis of within-session changes in reward contingencies, and data from the present experiments are consistent with this hypothesis. In addition, the fact that, like amphetamine, nicotine also induces release of catecholamines (including dopamine) suggests a possible shared mechanism for these effects (George et al. 2000; Rice and Cragg 2004).
Despite the robust effects of nicotine on probability discounting, there were no effects of nicotine on choice behavior in the delay discounting task. This lack of effect was surprising, as not only was there no evidence for perseverative behavior as in the probability discounting task, but several prior studies have reported that acute nicotine decreases choice of large delayed rewards (Dallery and Locey 2005; Kolokotroni et al. 2011; for similar results in a choice task involving risk of punishment as the reward cost, see also Mitchell et al. 2011; although see Anderson and Diller 2010). Considered together, these findings suggest that the perseveration-inducing effects of nicotine are limited to only some forms of cost–benefit decision making and that its effects in other choice tasks more strongly reflect its influences on other aspects of neurocognitive function. Indeed, similar dissociations across different forms of cost–benefit decision making have been observed following acute amphetamine administration (Cardinal et al. 2000; Simon et al. 2009).
One reported effect of nicotine administration which might account for the absence of apparent effects on delay discounting in this study is its actions on sensitivity to reward magnitude. Locey and Dallery (2009, 2011) recently conducted several experiments in decision making tasks in which they found that acute nicotine caused a shift in choice behavior from large to small rewards in the absence of effects on sensitivity to reward delay or probability. Such nicotine-induced decreases in sensitivity to reward magnitude could account for previous reports of greater impulsive choice in delay discounting tasks (Dallery and Locey 2005; Kolokotroni et al. 2011), as well as reports of greater impulsive choice in human smokers (Mitchell 1999). The fact that, in the present study, mecamylamine produced a trend toward increased choice of the large reward in both discounting tasks could also be consistent with a role for nicotinic receptors in reward sensitivity. It is not clear why nicotine failed to influence impulsive choice in the present study; however, given that the delay discounting task design used here may have been particularly susceptible to the effects of nicotine on perseverative behavior (unlike the designs used by Dallery and Locey (2005) and Kolokotroni et al. (2011), in which the order of presentation of the delays was not consistent), it may be the case that elevated perseveration (which would be expected to maintain choice of the large reward in the face of increasing delay costs) counteracted any decrease in choice of the large reward due to decreases in sensitivity to reward magnitude, resulting in no net change in choice behavior (although, admittedly, there was no direct evidence for such decreases in sensitivity to reward magnitude in the present study). Ultimately, the effects of acute nicotine on choice behavior in cost–benefit decision making tasks likely depend on the relative values of the large and small rewards and the degree to which different reward costs influence these values. In addition, baseline levels of reward choice in such tasks likely influence the magnitude and direction of such effects (Newhouse et al. 2004; Stanis et al. 2008). Future research employing parametric variation of these variables across subjects with varying degrees of baseline performance will be necessary to fully elucidate the contributions of the different effects of nicotine on choice behavior.
Muscarinic receptors and cost–benefit decision making
Acute administration of the muscarinic receptor agonist oxotremorine had no effect on choice behavior in either the probability or delay discounting tasks (although the behavioral efficacy of the drug was evident in the reduction in locomotor activity at the highest dose). In contrast, the muscarinic antagonist scopolamine produced robust decreases in choice of the large reward across both tasks, particularly at high doses. This effect of scopolamine, together with the lack of effect of oxotremorine, suggests that muscarinic signaling may be optimized for decision making (e.g., that muscarinic tone is high in neural systems responsible for choice behavior), such that an agonist (even one that has other behavioral effects, as it did on locomotion) will have minimal effects on choice behavior. However, with such optimization might come great sensitivity to disruption (e.g., high muscarinic tone might be sensitive to blockade), such that muscarinic antagonists would cause severe disruptions in performance. Additionally, the fact that the effects of scopolamine were qualitatively similar across tasks suggests that it exerts its effects on choice behavior through a common mechanism. One likely possibility is through effects on food motivational or consummatory processes. While the effects of systemic scopolamine administration on food motivation are mixed (Khokhlova et al. 2001; Plakke et al. 2008), scopolamine infused directly into the nucleus accumbens is reported to decrease food consumption (Perry et al. 2010; Pratt and Blackstone 2009). Such an effect is consistent with the increase in trial omissions produced by both muscarinic antagonists (i.e., reductions in food motivation via prefeeding have similar effects in these and other choice tasks; Simon et al. 2009; St. Onge and Floresco 2008), as well as with informal observations in the present experiments of occasional failures to consume all of the earned food pellets following scopolamine administration. The fact that the high dose of scopolamine reduced choice of the large reward during the first trial block (i.e., in the absence of risk of reward omission or delay to reward delivery) provides further support for the disruptive effects of (high dose) muscarinic blockade on some aspects of food motivation.
The disruptive effects of atropine on task performance were less evident. None of the lower doses of the drug affected choice behavior in the first block of either task, indicating relatively intact abilities to detect and respond appropriately to the different reward magnitudes. However, the higher doses of atropine used in the probability discounting task tended to “flatten” the discounting curves (reducing choice of the large reward at high probabilities of delivery and increasing choice of the large reward at low probabilities of delivery). This pattern of choice behavior could reflect truly impaired decision making (i.e., inability to use information regarding reward probability to guide behavior); however, this same pattern of “flattened” probability discounting curves has also been observed in this task following reductions in food motivation via prefeeding (St. Onge and Floresco 2008), suggesting that it could be linked to the effects of muscarinic antagonists on motivational processes. Interestingly, the adjusted high dose of atropine in the delay discounting task (which was the low dose used in the probability discounting task) appeared to increase impulsive choice without observable effects on motivation. This suggests that, although muscarinic receptors may play a critical role in food motivation, they may also be involved in processes more directly underlying impulsive choice.
Conclusions
The results of these experiments indicate that cholinergic receptors play multiple, dissociable roles in different forms of cost–benefit decision making. The findings concerning nicotinic receptors are of particular interest, as these receptors are a clinical target in several conditions which prominently feature alterations in cost–benefit decision making, including ADHD and addiction (Nakamura et al. 2007; Potter et al. 2006). Given the diversity of nicotinic receptor subtypes, it will be important in future studies to determine the roles of these subtypes (and the brain systems in which they act) in different aspects of cost–benefit decision making. In addition, given evidence that drugs targeting nicotinic receptors may exert different effects in individuals with different levels of baseline performance on cost–benefit decision making tasks (Potter et al. 2011), future research should also incorporate animal models of clinical conditions which capture features of altered cost–benefit decision making (e.g., elevated impulsivity in animal models of ADHD and addiction (Adriani et al. 2003; Setlow et al. 2009)). Ultimately, such research may prove useful for guiding development of cholinergic therapies for these conditions.
Acknowledgments
This research was supported by NIH grants MH65728 (IAM), AG029421 (JLB), and DA024671 (BS).
Footnotes
Conflicts of interest We have no conflicts of interest regarding the contents of this manuscript.
Contributor Information
Ian A. Mendez, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA, USA
Ryan J. Gilbert, Department of Neuroscience, University of Florida, Gainesville, FL, USA
Jennifer L. Bizon, Department of Neuroscience, University of Florida, Gainesville, FL, USA
Barry Setlow, Email: setlow@ufl.edu, Department of Psychiatry, University of Florida, P.O. Box 100256, Gainesville, FL 32610-0256, USA.
References
- Adriani W, Caprioli A, Granstrem O, Carli M, Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci Biobehav Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behav Pharmacol. 2010;21:754–764. doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W, Odum A, Madden G. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of D-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice on rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Evenden J, Ryan C. The pharmacology of impulsive behavior in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Corticolimbic-striatal circuits subserving different forms of cost–benefit decision making. Cognit Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- George TP, Verrico CD, Picciotto MR, Roth RH. Nicotinic modulation of mesoprefrontal dopamine neurons: pharmacologic and neuroanatomic characterization. J Pharmacol Exp Therapeut. 2000;295:58–66. [PubMed] [Google Scholar]
- Gleichgerrcht E, Ibáñez A, Roca M, Torralva T, Manes F. Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol. 2010;6:611–623. doi: 10.1038/nrneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- Ho MY, Mobini S, Chiang TJ, Bradshaw CM. Theory and method in the quantitative analysis of “impulsive choice” behavior: implications for psychopharmacology. Psychopharmacology. 1999;146:362–372. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology off motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Khokhlova VN, Marzhanova GK, Dolbakyan EE. The role of muscarinic cholinoceptors in the retrieval of an operant food-related conditioned reflex in cats. Nuerosci Behav Physiol. 2001;31:291–298. doi: 10.1023/a:1010334601800. [DOI] [PubMed] [Google Scholar]
- Killeen PR. Models of trace decay, eligibility for reinforcement, and delay of reinforcement gradients, from exponential to hyperboloid. Behavioral Processes. 2011;87:57–63. doi: 10.1016/j.beproc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioral disinhibition in rats. Psychopharmacology. 2011;217:455–473. doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- Locey ML, Dallery J. Isolating behavioral mechanisms of inter-temporal choice: nicotine effects on delay discounting and amount sensitivity. J Exp Anal Behav. 2009;91:213–223. doi: 10.1901/jeab.2009.91-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locey ML, Dallery J. Nicotine and the behavioral mechanisms of intertemporal choice. Behav Process. 2011;87:18–24. doi: 10.1016/j.beproc.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting amount procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analysis of behavior: the effects of delay and of intervening events on reinforcement value. Erlbaum; Hillsdale: 1987. pp. 55–73. [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124:470–477. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology. 2011;218:703–712. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Mendez IA, Vokes CM, Damborsky JC, Winzer-Serhan UH, Setlow B. Effects of developmental nicotine exposure in rats on decision making in adulthood. Behav Pharmacol. 2012;23:34–42. doi: 10.1097/FBP.0b013e32834eb04a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. J Nucl Med. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an [alpha]4[beta]2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- O’Hare E, Weldon DT, Bettin K, Cleary J, Mach JR. Serum anticholinergic activity and behavior following atropine sulfate administration in the rat. Pharmacol Biochem Behav. 1997;56:151–154. doi: 10.1016/S0091-3057(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Perry ML, Andrzejewski ME, Bushek SM, Baldo BA. Intra-accumbens infusion of a muscarinic antagonist reduces food intake without altering the incentive properties of food associated cues. Behav Neurosci. 2010;124:44–54. doi: 10.1037/a0018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakke B, Ng C, Poremba A. Scopolamine impairs auditory delayed matching-to-sample performance in monkeys. Neurosci Lett. 2008;438:126–130. doi: 10.1016/j.neulet.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A, Newhouse P. Cognitive effects of acute nicotine and ultra low-dose mecamylamine in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:69s. [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA, Bucci DJ. Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res. 2006;175:201–211. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Potter AS, Ryan KK, Newhouse PA. Effects of acute ultra-low dose mecamylamine on cognition in adult attention-deficit/hyper-activity disorder (ADHD) Hum Psychopharmacol. 2009;24:309–317. doi: 10.1002/hup.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Bucci DJ, Newhouse PA. Manipulation of nicotinic acetylcholine receptors differentially affects behavioral inhibition in human subjects with and without disordered baseline impulsivity. Psychopharmacology. 2011;220:331–340. doi: 10.1007/s00213-011-2476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WE, Blackstone K. Nucleus accumbens acetylcholine and food intake: decreased muscarinic tone reduces feeding but not food-seeking. Behav Brain Res. 2009;198:252–257. doi: 10.1016/j.bbr.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Richards J, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioral Processes. 2004;64:333–344. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Rice M, Cragg S. Nicotine amplified reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Setlow B, Mendez IA, Mitchell MR, Simon NW. Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav Pharmacol. 2009;20:380–389. doi: 10.1097/FBP.0b013e3283305eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2008;34:1–17. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- St Onge J, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cerebr Cortex. 2009;20:1816–1828. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- St Onge J, Chiu Y, Floresco SB. Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology. 2010;211:209–221. doi: 10.1007/s00213-010-1883-y. [DOI] [PubMed] [Google Scholar]
- Stanis J, Marquez Avila H, White M, Gulley J. Dissociation between long-lasting behavioral sensitization to amphetamine and impulsive choice in rats performing a delay-discounting task. Psychopharmacology. 2008;199:539–548. doi: 10.1007/s00213-008-1182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Molina B, Pelham W, Gnagy E. Risky driving in adolescents and young adults with childhood ADHD. J Pediatr Psychol. 2007;32:745–759. doi: 10.1093/jpepsy/jsm002. [DOI] [PubMed] [Google Scholar]
- Warpman U, Nordberg A. Epibatidine and ABT 418 reveal selective losses of alpha 4 beta 2 nicotinic receptors in Alzheimer brains. NeuroReport. 1995;6:2419–2423. doi: 10.1097/00001756-199511270-00033. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Bellebaum C, Brüne M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009;23:571–580. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- Xie X, Arguello AA, Reittinger AM, Wells AM, Fuchs RA. Role of nicotinic acetylcholine receptors in the effects of cocaine-paired contextual stimuli on impulsive decision making in rats. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S, Sacco K, George T, Potenza M. Risk/reward decision-making in schizophrenia: a preliminary examination of the influence of tobacco smoking and relationship to Wisconsin Card Sorting Task performance. Schizophr Res. 2009;110:156–164. doi: 10.1016/j.schres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]