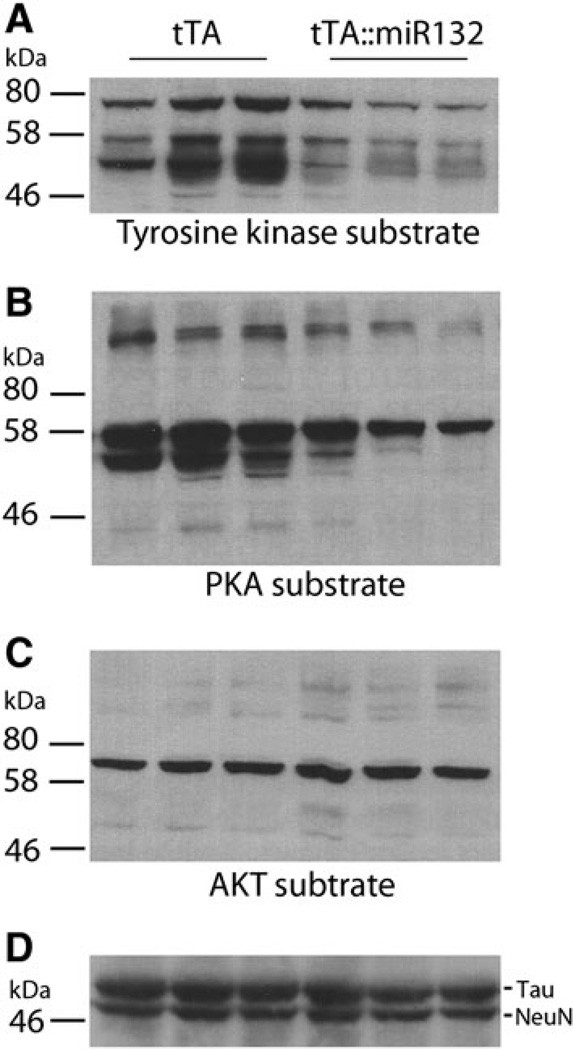
Fig. 6.
tTA::miR-132 mice exhibit altered kinomic activity. To begin to identify molecular mechanisms by which miR-132 influences cognitive capacity, hippocampal lysates from tTA and tTA::miR-132 animals were probed with phospho motif-specific antibodies that infer the activation state of protein tyrosine kinases (a, tyrosine kinase substrate), protein kinase A (b PKA substrate), and AKT (c AKT substrate). a Transgenic animals showed decreased phosphorylation of the tyrosine kinase substrate motif and b the PKA substrate motif (RRXS*/T*: * denotes phosphorylated amino acid residue) relative to tTA mice. Phosphorylation of the AKT motif (RXXS*/T*) remained relatively unaffected in tTA::miR-132 mice. As a protein loading control, membranes were also probed for Tau and NeuN; for clarity, only a subset of the banding pattern is presented. Lysates from three animals of each genotype were profiled (i.e., biological triplicate determinations)