Abstract
OBJECTIVES
The aims of this paper are to: 1) describe objectively-confirmed physical activity patterns across three chronic cardiopulmonary conditions, and 2) examine the relationship between selected physical activity dimensions with disease severity, self-reported physical and emotional functioning, and exercise performance.
INTERVENTIONS
Not applicable.
DESIGN
Cross-sectional study.
SETTING
Participant’s home environment.
PARTICIPANTS
Patients with cardiopulmonary illnesses: chronic obstructive pulmonary disease (COPD, n=63), heart failure (HF, n=60), and patients with implantable cardioverter defibrillator (ICD, n=60).
MAIN OUTCOME MEASURES
Seven ambulatory physical activity dimensions (total steps, percent time active, percent time ambulating at low, medium, and high intensity, maximum cadence for 30 continuous minutes, and peak performance) were measured with an accelerometer.
RESULTS
Subjects with COPD had the lowest amount of ambulatory physical activity compared to subjects with heart failure and cardiac dysrhythmias (all seven activity dimensions, p<.05); total step counts were: 5319 vs. 7464 vs. 9570, respectively. Six minute walk distance were correlated (r=.44 to .65, p<.01) with all physical activity dimensions in the COPD sample, the strongest correlations being with total steps and peak performance. In subjects with cardiac impairment, maximal oxygen consumption had only small to moderate correlations with five of the physical activity dimensions (r=.22 to .40, p<.05). In contrast, correlations between six minute walk test distance and physical activity were higher (r=.48 to .61, p<.01) albeit in a smaller sample of only patients with heart failure. For all three samples, self-reported physical and mental health functioning, age, body mass index, airflow obstruction, and ejection fraction had either relatively small or non-significant correlations with physical activity.
CONCLUSIONS
All seven dimensions of ambulatory physical activity discriminated between subjects with COPD, heart failure, and cardiac dysrhythmias. Depending on the research or clinical goal, use of one dimension such as total steps may be sufficient. Although physical activity had high correlations with performance on a six minute walk test relative to other variables, accelerometry-based physical activity monitoring provides unique, important information about real-world behavior in patients with cardiopulmonary illness not already captured with existing measures.
Keywords: ambulatory physical activity, walking, monitoring, COPD, heart failure, implantable defibrillator, exercise performance
Cardiopulmonary diseases are the leading causes of morbidity and mortality worldwide.1 Epidemiological studies based on self-reported physical activity show that higher levels of activity are associated with lower risk of incident chronic obstructive pulmonary disease (COPD) in smokers and in patients with COPD, decreased risk of hospital admissions, exacerbations and mortality.2–4 A recent 4-year prospective study of 170 patients with COPD showed that objectively measured physical activity was the best predictor of all-cause mortality when compared with a broad range of other prognostic factors including airflow obstruction, exercise performance, cardiovascular status, nutritional and muscular status, systemic inflammation, health status, depressive symptoms, and dyspnea. Each increase of 1845 steps per day was associated with a 51% lower risk of death (HR, 0.49; 95% CI, 0.35–0.69).5 The physiological processes underlying the relationship between physical activity and survival are complex and only incompletely understood. However, it has been hypothesized that inactivity leads to cellular and molecular dysregulation, which directly contributes to the development of multiple chronic conditions.6, 7
Similarly, associations have been found for self-reported physical activity with the primary and secondary prevention of cardiovascular diseases in a number of epidemiological studies.6–9 However, far fewer studies have been published on objectively measured physical activity in select cardiac populations such as heart failure10–12, severe cardiac dysrhythmias, or coronary artery disease to provide useful benchmarks of physical activity levels for comparisons across studies. Activity monitoring based on accelerometry can more precisely capture what patients actually do in their daily lives instead of what they report or what they are capable of with laboratory exercise testing.13 While the pathophysiological processes in the development of COPD, heart failure, and cardiac rhythm disorders differ, we posit that decreased physical activity is a common pathway to impaired functioning and disability in these and other chronic conditions14 and that objective assessment of physical activity provides a unique but universal metric for comparison across diseases and studies. Therefore, the aims of this paper are to: 1) describe objectively-confirmed physical activity patterns across three chronic cardiopulmonary conditions, and 2) examine the relationship between selected physical activity dimensions with disease severity, self-reported physical and emotional functioning, and exercise performance.
METHODS
Participants
Between 2007 and 2010, a combined convenience sample of 183 outpatients at a Veterans Administration and university medical center were selected for study from the combined databases of three similar outpatient studies of activity patterns in cardiopulmonary illness.15, 16 All subjects had either a diagnosis of COPD, a history of life-threatening cardiac arrhythmias, or heart failure and had been clinically stable for at least one month and under optimal medical management. All subjects were able to read, speak, and write English and were not carrying out more than two days of supervised exercise per week. Subjects were excluded if they had less than one year to live, active malignancy, hypoxia with exertion (oxygen saturation <86% during exercise testing), significant psychiatric illness or recent drug abuse that would impair participation, and neuromuscular disease that limited daily activity. In addition, subjects were excluded if they evidenced disease exacerbation, uncontrolled cardiac dysrhythmias, unstable angina, recent myocardial infarction (MI), or cardiothoracic surgery within the past three months. All three studies were approved by their respective institutional review boards and were registered with ClinicalTrials.gov (NCT00373932; NCT00522340, NCT00467298).
COPD Sample (N=63): The COPD subjects had to have at least mild COPD (GOLD Stage I) defined as a post-bronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio <0.70 with FEV1 >80% predicted with daily activities limited by dyspnea.
Heart Failure Sample (N=60): In addition to the general inclusion and exclusion criteria described earlier, heart failure subjects had to have an ejection fraction (EF) of ≤ 0.3517, 18 and daily activities limited by dyspnea or fatigue.
Cardiac Dysrhythmia Sample (N=60): In addition to the general inclusion and exclusion criteria, the cardiac dysrhythmia sample had a history of life-threatening dysrhythmia that required the placement of an implantable cardioverter defibrillator (ICD) for secondary prevention of sudden cardiac arrest. These subjects were also on beta blockers.
Measurements
Demographics data included self-reported age, gender, education and marital status.
Health Status included self-report of chronic conditions (Charlson co-morbidity index), ejection fraction (EF) obtained from medical records, and spirometry which was performed according to American Thoracic Society (ATS) standards using a Koko spirometer.a Post bronchodilator values were used.
Exercise Performance was assessed using the six minute walk test (6MWT)19 and a modified Balke treadmill symptom-limited test protocol.20 Participants performed two 6MWT according to ATS guidelines and the longer of the two tests was used for analysis. Maximum oxygen consumption (V02max) was measured during the cardiopulmonary exercise test session in an exercise laboratory and determined as the average value observed over the last 10 seconds of exerciseb.
Ambulatory Physical Activity was measured using a pager-sized, lightweight, Stepwatch™ 3 Activity Monitorc fastened above the right ankle. The SAM is a dual-axis accelerometer linked to a microprocessor sensor that directly and continuously records gait cycles (strides) based on acceleration, position, and timing information. Stride counts are doubled to represent steps. The SAM has been validated for use in healthy and chronically ill older adults in laboratory and community settings and has an accuracy of 98–99%.21, 22 Participants were asked to wear the SAM during waking hours for seven days at baseline. The SAM was programmed to record in 1-minute epochs; a valid day was defined as having 10 or more hours (600 mins.) of monitor wear.
The Stepwatch software was used to produce the following physical activity dimensions: (1) total daily steps taken, percent time active, percent time spent ambulating at low intensity (1–30 steps/min), medium intensity (31–80 steps/min), and high intensity (≥ 80 steps/min), (2) max cadence for 30 continuous minutes which provides a proxy of walking intensity during a typical recommended bout of endurance exercise, and (3) peak performance which represents short walking bursts and is obtained by ranking all minutes of the day according to cadence, and then averaging the highest 30 values. These variables were calculated for each day, and then the daily values were averaged over the total monitoring period.
Health related quality of life (HRQL) was measured with the Medical Outcomes Study Short-Form 36 (MOS-SF36).23 The SF-36 produces two composite scales of physical and mental functioning with higher scores indicating better HRQL.
Data Analysis
Analysis of variance or Fischer’s Exact tests were used to compare demographic, disease severity, functioning, and physical activity patterns across groups. Pearson correlation coefficients were computed for the bivariate correlations. All analyses were conducted using SPSS 15.0d. A p value <.05 was considered statistically significant.
RESULTS
Sample Characteristics
The COPD sample was the oldest with a mean age of 67. A majority of the total sample was obese Caucasian males with at least some college education (Table I). Although the heart failure sample had the highest chronic disease burden, their self-reported physical functioning score was better than the COPD sample. The younger sample with cardiac dysrhythmias had the worst mental health functioning score despite lower chronic disease burden and the highest physical functioning score of the three groups. The 6MWT was not significantly different between the COPD and heart failure sample. As expected, VO2max was significantly different between patients with heart failure and cardiac dysrhythmias.
Table I.
Subject Characteristics
| COPD | Heart Failure | Cardiac Dysrhythmias | Total | ANOVA* | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 63 | n = 60 | n = 60 | n = 183 | p | |||||
| Age | 67.0. | (9.3) | 60.5 | (10.8) | 55.4 | (11.6) | 61.1 | (11.6) | <.001†, ‡, § |
| Gender (Male) | 58 | (91%) | 57 | (95%) | 44 | (72%) | 159 | (86%) | <.001‡, § |
| Ethnicity (Caucasian) | 53 | (83%) | 48 | (80%) | 58 | (95%) | 159 | (86%) | .04†, ‡, § |
| Education (At least some college) | 31 | (48%) | 41 | (73%) | 44 | (72%) | 119 | (64%) | .006†, ‡ |
| Body Mass Index (BMI) | 29.7 | (7.0) | 30.5 | (7.0) | 29.0 | (6.0) | 29.7 | (6.7) | .46 |
| Charlson Comorbidity Index | 1.6 | (1.2) | 2.3 | (1.3) | 1.0 | (0.9) | 1.6 | (1.2) | <.001†, ‡, § |
| Ejection Fraction | 55% | (8) [n=25] | 27% | (7) | 49% | (11) | 41% | (15) | <.001†, § |
| Spirometry | |||||||||
| FEV1 % Predicted | 36 | (15) | 72 | (20) | 85 | (15) | 64 | (27) | <.001†, ‡, § |
| FVC % Predicted | 57 | (16) | 73 | (17) | 85 | (14) | 72 | (20) | <.001†, ‡, § |
| FEV1/FVC | 0.46 | (0.16) | 0.73 | (0.11) | 0.73 | (0.08) | 0.64 | (0.17) | <.001†, ‡ |
| Health Related Quality of Life (HRQL) | |||||||||
| SF-36 Physical Component Score | 30.7 | (7.7) | 42.7 | (11.8) | 51.2 | (9.0) | 41.4 | (12.8) | <.001†, ‡, § |
| SF-36 Mental Component Score | 45.3 | (13.0) | 43.4 | (8.6) | 40.7 | (6.3) | 43.1 | (9.9) | .035 |
| Exercise Performance | |||||||||
| Six Minute Walk (meters) | 340.2 | (113.1) | 385.2 | (104.4) [n=24] | --- | --- | 353.6 | (112) | .094 |
| VO2 Max (mL/min/kg) | --- | --- | 20.3 | (5.3) [n=36] | 24.4 | (6.1) | 22.8 | (6.1) | .001 |
Values shown are mean(sd) or count(%)
Abbreviations: EF: Ejection Fraction; FEV1 = Forced Expiratory Volume in 1 second; FVC = Forced V Capacity; VO2Max: Maximum oxygen consumption
Overall group comparison; Pairwise comparisons with Bonferroni corrections: p<.05,
COPD vs. Heart Failure,
COPD vs. Cardiac Dysrhythmias, and
Heart Failure vs. Cardiac Dysrhythmias
Physical Activity Patterns
Stepwatch recordings were available for a median of 5 days (range: 3 to 18 days), a sufficient duration for cross-sectional descriptions of physical activity patterns.24, 25 The COPD sample engaged in the lowest amount of ambulatory physical activity compared to the two cardiac samples-total daily steps were 5319 vs. 7464 vs. 9570, p<.001 (Table II). The total daily step count for subjects with cardiac dysrhythmias was comparable to that of healthy adults.26, 27 The COPD subjects also spent the lowest percentage of time being active but this was only significantly different from subjects with cardiac dysrhythmias (32% vs. 38%, p<.05). The two cardiac samples spent a similar percentage of time in medium intensity walking activity (25% and 27%) and were both significantly higher than the COPD sample (22%). Subjects with COPD spent most of their active time in low intensity activity (76%) in contrast to subjects with cardiac dysrhythmias who spent 66% of their time in low intensity activity. Peak performance was significantly different across groups with the cardiac dysrhythmias sample having the highest step rate. MaxSteps30 which captures the highest step rate in 30 minutes and is a proxy for walking intensity during a typical recommended bout of endurance exercise showed that subjects with COPD performed only 51% of their walking capacity (peak performance) compared to 61–66% in the cardiac samples.
Table II.
Ambulatory Physical Activity Across Cardiopulmonary Diseases
| COPD | Heart Failure | Cardiac Dysrhythmias | Total | F Statistic | ANOVA* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 63 | n = 60 | n = 60 | n = 183 | p | ||||||
| Total Steps | 5319 | (2712) | 7464 | (3724) | 9570 | (3622) | 7416 | (3783) | 24.4 | <.001†, ‡, § |
| % Time Active | 32 | (14) | 35 | (12) | 38 | (11) | 35 | (13) | 4.1 | .015 ‡ |
| % Time High Intensity (> 80steps/min) | 2 | (2) | 5 | (5) | 7 | (5) | 4 | (5) | 19.4 | <.001†, ‡, § |
| % Time Med Intensity (31–80 steps/min) | 22 | (8) | 25 | (8) | 27 | (6) | 25 | (8) | 9.5 | <.001†, ‡ |
| % Time Low Intensity(≤30 steps/min) | 76 | (9) | 70 | (10) | 66 | (8) | 71 | (10) | 22.4 | <.001†, ‡, § |
| Peak Performance (steps/min) | 57 | (16) | 71 | (20) | 85 | (18) | 71 | (21) | 38.4 | <.001†, ‡, § |
| Max Steps 30 (steps/min) | 28 | (12) | 43 | (23) | 57 | (24) | 43 | (23) | 26.9 | <.001†, ‡, § |
Values shown are mean(sd)
Overall group comparison; Pairwise comparisons with Bonferroni corrections: p<.05,
COPD vs. Heart Failure,
COPD vs. Cardiac Dysrhythmias, and
Heart Failure vs. Cardiac Dysrhythmias
Bivariate Correlations
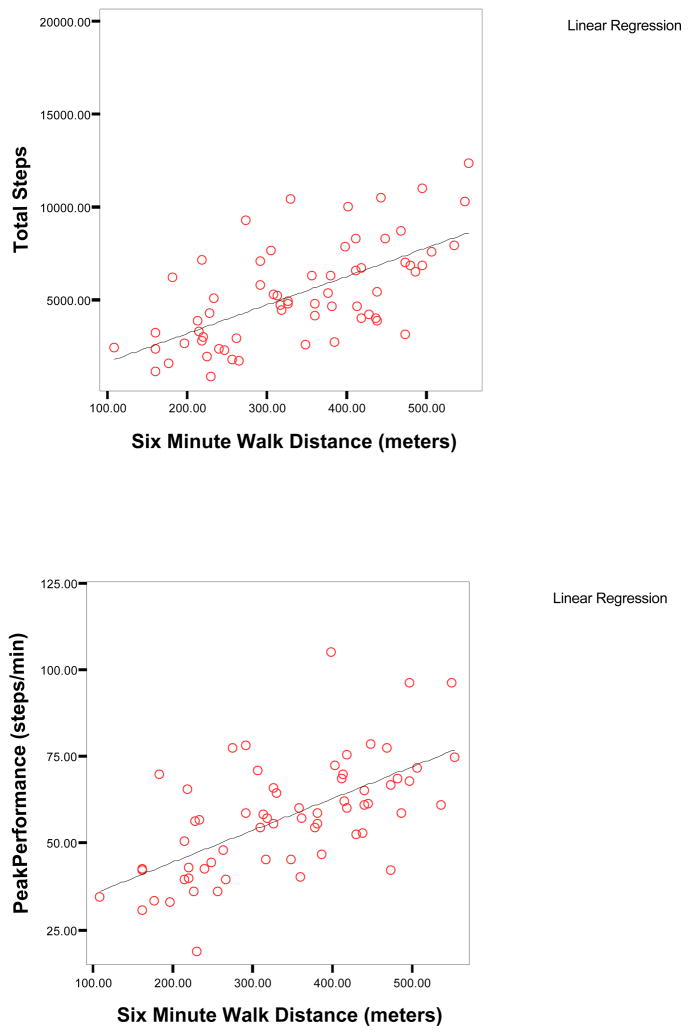
In the COPD sample, six minute walk distance had moderate to high correlations (r=.44 to .65, p<.01) with all seven physical activity dimensions, the strongest correlations being with total steps and peak performance (Table III & Figure IA). Airway obstruction (FEV1% predicted) had small to moderate correlations with all (r=.30 to −.45, p<.01) but one dimension of physical activity, percent time active. Self-reported physical functioning was correlated only with peak performance (r=.27, p<.05). Age, BMI and mental health functioning were not significantly correlated with any of the physical activity dimensions whereas higher chronic disease burden was associated with less time spent in high intensity walking activity (r=−.26, p<.05).
Table III.
Correlations Between Physical Activity Dimensions with Demographics, Health Status, and Exercise Performance in Patients with COPD (n=63)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.Total Steps | 1.00 | |||||||||||||
| 2. % Time Active | .85¶ | 1.00 | ||||||||||||
| 3. % Time High Intensity | .53¶ | .25|| | 1.00 | |||||||||||
| 4. % Time Medium Intensity | .63¶ | .32|| | .30|| | 1.00 | ||||||||||
| 5. % Time Low Intensity | −.68¶ | −.33‡ | −.63‡ | −.98‡ | 1.00 | |||||||||
| 6. Peak Performance | .87¶ | .62¶ | .80¶ | .70¶ | −.80¶ | 1.00 | ||||||||
| 7. Max Steps 30 | .79¶ | .60¶ | .75¶ | .54¶ | −.65¶ | .90¶ | 1.00 | |||||||
| 8. 6MW Distance | .64¶ | .51¶ | .47¶ | .53¶ | −.57¶ | .65¶ | .44¶ | 1.00 | ||||||
| 9. Age | −.22 | −.19 | −.09 | −.11 | .12 | −.13 | −.07 | −.22 | 1.00 | |||||
| 10. BMI | .02 | −.08 | .09 | .22 | −.21 | .08 | .13 | −.21 | −.09 | 1.00 | ||||
| 11. FEV1%Pred | .30|| | .15 | .44¶ | .40¶ | −.45¶ | .43¶ | .39¶ | .17 | .10 | .41¶ | 1.00 | |||
| 12. Co-Morbidity | −.17 | −.09 | −.26| | −.15 | .19 | −.17 | −.12 | −.22 | .23 | .23 | −.10 | 1.00 | ||
| 13. SF-36 PCS | .18 | .15 | .28 | .18 | −.22 | .27|| | .22 | .41¶ | .03 | −.06 | .01 | .04 | 1.00 | |
| 14. SF-36 MCS | −.06 | −.15 | .19 | −.06 | .00 | .04 | .07 | −.02 | .22 | −.01 | .06 | .09 | .17 | 1.00 |
p< 0.05,
p<0.01
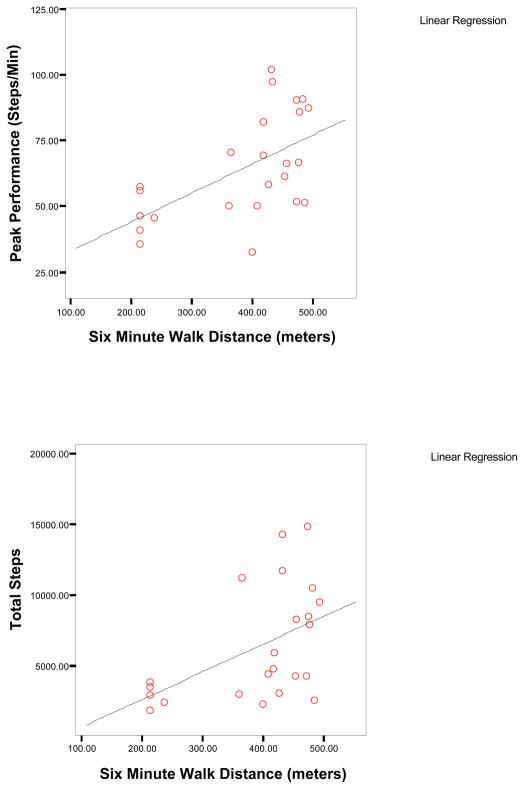
Figure I.
Figure IA. Scatterplots of Six Minute Walk Distance, Total Steps, and Peak Performance in Subjects with COPD (n=63)
Figure IB. Scatterplots of Six Minute Walk Distance, Total Steps, and Peak Performance in Subjects with Heart Failure (n=24)
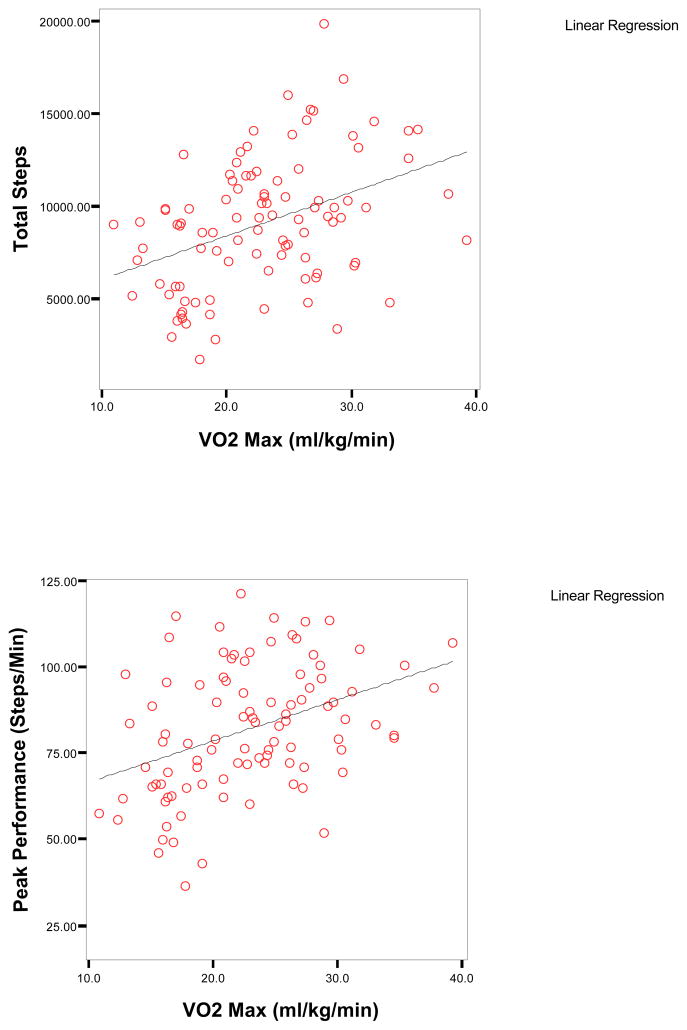
Maximal oxygen consumption had only small to moderate correlations with five of the seven physical activity dimensions (r=.22 to .40, p<.05) in the combined cardiac sample (Table IV); the strongest correlations were with total steps and peak performance (Figure IB). In contrast, correlations between distance covered on a six minute walk test with five physical activity dimensions were higher (r=.48 to .61, p<.01) albeit in a smaller sample of only patients with heart failure. Higher comorbidity was associated with less physical activity (r=−0.27 to −0.44, p<.01) but ejection fraction was only correlated with time spent in high intensity walking activity (r=.19) and peak performance (r=.26). Self-reported physical functioning had small to moderate correlations with six of the seven physical activity dimensions (r=.22 to .37, p<.05). Age, FEV1% predicted, BMI and mental health functioning were not significantly correlated with any of the physical activity dimensions with the exception of percent time active and BMI.
Table IV.
Correlations Between Physical Activity Dimensions with Demographics, Health Status, and Exercise Performance in Patients with Heart Failure and Cardiac Dysrhythmias (n=96)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Total Steps | 1.00 | |||||||||||||||
| 2. % Time Active | .78¶ | 1.00 | ||||||||||||||
| 3. % Time High Intensity | .45¶ | .01 | 1.00 | |||||||||||||
| 4. % Time Medium Intensity | .50¶ | .35¶ | −.03 | 1.00 | ||||||||||||
| 5. % Time Low Intensity | −.68¶ | −.27¶ | −.64¶ | −.75¶ | 1.00 | |||||||||||
| 6. Peak Performance | .74¶ | .39¶ | .82¶ | .22|| | −.71¶ | 1.00 | ||||||||||
| 7. Max Steps 30 | .69¶ | .33¶ | .83¶ | .15 | −.66¶ | .91¶ | 1.00 | |||||||||
| 8. VO2 Max | .40¶ | .34¶ | .22|| | .06 | −.19 | .39¶ | .31¶ | 1.00 | ||||||||
| 9. 6MWD (n=24) | .52¶ | .59¶ | .48|| | .25 | −.38 | .58¶ | .61¶ | -- | 1.00 | |||||||
| 10. Age | −.09 | −.14 | .03 | −.02 | .00 | −.08 | .05 | −.41¶ | −.26 | 1.00 | ||||||
| 11. BMI | −.18 | −.25|| | −.07 | .02 | .03 | −.19 | −.16 | −.45¶ | −.06 | .15 | 1.00 | |||||
| 12. FEV1%Pred | .19 | .17 | .03 | .15 | −.13 | .13 | .10 | .34¶ | .41 | .03 | −.09 | 1.00 | ||||
| 13. EF | .12 | .01 | .19|| | −.01 | −.12 | .26¶ | .16 | .33¶ | .10 | −.16 | −.07 | .22|| | 1.00 | |||
| 14. Comorbidity | −.35¶ | −.29¶ | −.27¶ | −.14 | .29¶ | −.44¶ | −.36¶ | −.51¶ | −.36 | .30¶ | .24|| | −.12 | −.42¶ | 1.00 | ||
| 15. SF-36 PCS | .35¶ | .21 | .23|| | .22|| | −.32¶ | .37¶ | .27¶ | .29¶ | .39 | −.02 | .06 | .14 | .11 | −.18 | 1.00 | |
| 16. SF-36 MCS | .03 | .07 | −.01 | .00 | .01 | −.02 | −.05 | −.01 | .15 | .16 | .08 | .12 | −.15 | .19 | .19 | 1.00 |
p< 0.05,
p< 0.01
DISCUSSION
The primary finding from this study which used a highly accurate ankle mounted accelerometer showed that all dimensions of ambulatory physical activity discriminated between subjects with COPD, heart failure, and cardiac dysrhythmias. Specifically, subjects with COPD engaged in the lowest volume of ambulatory physical activity followed by subjects with heart failure, and cardiac dysrhythmias. To the best of our knowledge, we are not aware of any other published reports that have compared physical activity patterns across three cardiopulmonary conditions. In both subjects with COPD and cardiac impairment, all seven physical activity dimensions had the highest correlations with distance covered on a six minute walk test; smaller, less consistent associations were found between physical activity and self-reported physical functioning and airway obstruction in the COPD sample and self-reported functioning, ejection fraction, and maximum oxygen consumption in patients with cardiac impairment. In addition, age, body mass index and mental health functioning were not related to any of the physical activity dimensions for all subjects. These collective findings suggest that physical activity as measured by an accelerometer provides unique, important information about real-world behavior in patients with COPD, heart failure, and cardiac dysrhythmias not already captured with existing instruments.
Tudor-Locke et al.28 established pedometer-determined physical activity cut points for healthy adults as: <2500 steps/day (basal physical activity), 2500 to <5000 steps/day (limited physical activity), 5000-<7500 (low activity), 7500 to <10,000 (somewhat active), 10,000 to <12,500 (active), and >12,500 (highly active). Recently, a review of 28 studies that included older adults aged 50–94 showed mean pedometer-determined physical activity ranged from 2,015 steps/day to 8,938 steps/day.29 Based on these classifications, our COPD sample is considered to be in the low activity category but well within the range of expected step counts for older adults. Earlier studies of patients with COPD that used different activity devices showed similar total step counts and distribution of activity intensity as our study.13, 25, 30–32
Since this is the first study to report physical activity patterns in patients with cardiac dysrhythmias, we did not have any benchmark to compare our findings. We were surprised to find the relatively high total step count in subjects with cardiac dysrhythmias. Tudor-Locke et al.’s classification would place them in the “somewhat active” to “active” category28 and having the highest step count in comparison to ten other chronic conditions.33 Their younger age, limited physical impairment and few comorbid conditions partly explain their ability to engage in a higher volume of physical activity, i.e., more steps, active time, and time spent in high and moderate intensity walking activity. The total step count for subjects with heart failure is similar to one other published study that had a higher percentage of women in the sample compared to our study.12
It is interesting to note that MaxSteps30 which is a proxy for continuous ambulatory exercise intensity over a 30 minute interval was as low as 51% (COPD sample) to 67% (cardiac dysrhythmias sample) of the peak performance step rate. These data suggest that patients with cardiopulmonary illnesses likely engage in only low to moderate intensity walking exercises when they are unsupervised. Two recent studies of patients with COPD that measured either total energy expenditure using an arm-mounted device or oxygen uptake during five self-paced activities of daily living using a portable metabolic cart found that patients use a high proportion of their peak aerobic capacity (55% to 85% of O2 peak) to perform daily activities.34, 35. If indeed patients with COPD are already exerting themselves at close to peak capacity with routine domestic ADLs, it is only understandable that they may not have sufficient reserve and/or desire to perform their independent walking exercises at a higher intensity. Further research is needed to understand this relationship between metabolic load experienced during domestic ADLs and exercise intensity.
Although the highest correlations between the seven physical activity dimensions were with distance walked on a six minute walk test, these correlations were not as high as a previous study of COPD patients which used an older model accelerometer that measured activity in vector magnitude units (r=.74).36 The finding of only small correlations between VO2max with five of the seven activity dimensions in this study is in contrast to findings by Jehn et al.12, 37 where a different activity monitor and cycle ergometer protocol were used. The correlation between total time walked and VO2max was 0.72 in that study. Differences in the magnitude of associations between various measures of laboratory exercise performance and physical activity across studies could also partly be due to the stability of the estimates and degree of variability in the correlates. Nevertheless, the consistent small to moderate correlations between the seven physical activity dimensions with self-reported physical functioning and disease severity across the three samples suggest that the measurement of actual physical activity behavior provides additional valuable information on functional impairments associated with cardiopulmonary illnesses and may also contribute to better prediction of survival in these clinical populations.5
Study Limitations
There are several limitations to this descriptive study. A limitation of the Stepwatch, similar to most other activity monitors on the market, includes the inability to measure activities of the upper extremity and other activities such as bicycling. While lower limb activity cannot be substituted for estimates of total energy expenditure, it is clearly a major determinant of energy expenditure (r=0.92) and in most circumstances, is an acceptable surrogate13. Because the combined data set emanated from three clinical intervention studies that had specific inclusion and exclusion criteria, the findings may not be generalizable to the broader population of patients with cardiopulmonary illnesses who are more severely affected by their condition and were unable to participate in the clinical trials or were insufficiently active to benefit from an exercise intervention. The COPD and heart failure samples were comprised mostly of men thus the findings may not extend to women; in addition, the heart failure sample was relatively small Seasonal variations may affect physical activity patterns.38 Baseline measurements across the three RCTS were performed throughout the year though this may not fully account for the variability in the activity patterns.
CONCLUSIONS
Findings from this study provide a useful benchmark of physical activity patterns in individuals with cardiopulmonary illness for comparison with future studies. All seven dimensions of ambulatory physical activity discriminated between subjects with COPD, heart failure, and cardiac dysrhythmias. Depending on the research or clinical goal, use of one dimension such as total steps may be sufficient. Although physical activity had high correlations with performance on a six minute walk test relative to other variables, accelerometry-based physical activity monitoring provides unique, important information about real-world behavior in patients with cardiopulmonary not already captured with existing measures.
Figure II.
Scatterplots of VO2Max, Total Steps, and Peak Performance in Subjects with Heart Failure and Cardiac Dysrhythmias (n=96)
Acknowledgments
Supported in part by: 1KL2RR025015-01 Nguyen, R01 NR008938 Carrieri-Kohlman; R01 HL 084550 Dougherty; HSR&D NRI 04-242 Dougherty and Steele
Role of the funding source
The study sponsor played no role in the study design, data collection, analysis and interpretation of data, and writing of the manuscript
ABRREVIATIONS
- COPD
Chronic obstructive pulmonary disease
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- EF
Ejection fraction
- ICD
Implantable cardioverter defibrillator
- ATS
American Thoracic Society
- 6MWT
Six minute walk test
- SAM
Stepwatch activity monitor
- HRQL
Health related quality of life
- VO2 max
Maximal oxygen consumption
Footnotes
Pulmonary Data Services; Louisville, CO
Viasys VMax series 229, Sensor Medics, San Diego, CA, USA
Stepwatch™ Orthocare Innnovations, 840 Research Parkway, Suite 200, Oklahoma City, OK 73104
SPSS Inc, Chicago, IL
Clinical Trials Registration: NCT00373932; NCT00522340, NCT00467298
Author contributions: Conception and design of the study: Nguyen, Steele, Dougherty
Acquisition of data, analysis and interpretation of data: Nguyen, Steele, Dougherty, Burr
Drafting the article or revising it critically for important intellectual content: Nguyen, Steele, Dougherty, Burr
Final approval of the version to be submitted: Nguyen, Steele, Dougherty, Burr
Reprints will not be available from the corresponding author
Conflict of interest
We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated. We certify that all financial and material support for this research and work are clearly identified in the title page of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Huong Q. Nguyen, University of Washington.
Bonnie G. Steele, Heath Services Research & Development VA Puget Sound Health Care System.
Cynthia M. Dougherty, University of Washington, Nurse Practitioner, VA Puget Sound Health Care System.
Robert Burr, University of Washington.
References
- 1.Global status report on noncommunicable diseases 2010: Description of the global burden of NCDs, their risk factors and determinants. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 2.Garcia-Aymerich J, Farrero E, Felez MA, Izquierdo J, Marrades RM, Anto JM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003 Feb;58(2):100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006 Sep;61(9):772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007 Mar 1;175(5):458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 5.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with chronic obstructive pulmonary disease: a prospective cohort study. Chest. 2011 Jan 27;140(2):331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003 Jun 24;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 7.Services USDoHaH. Physical Activity Guidelines Advisory Committee Report. Washington, DC: 2008. [Google Scholar]
- 8.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002 Sep 5;347(10):716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 9.Tanasescu M, Leitzmann MF, Rimm EB, Hu FB. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003 May 20;107(19):2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 10.van den Berg-Emons H, Bussmann J, Balk A, Keijzer-Oster D, Stam H. Level of activities associated with mobility during everyday life in patients with chronic congestive heart failure as measured with an “activity monitor”. Phys Ther. 2001 Sep;81(9):1502–1511. [PubMed] [Google Scholar]
- 11.Jehn M, Schmidt-Trucksaess A, Schuster T, et al. Accelerometer-based quantification of 6-minute walk test performance in patients with chronic heart failure: applicability in telemedicine. J Card Fail. 2009 May;15(4):334–340. doi: 10.1016/j.cardfail.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Jehn M, Schmidt-Trucksass A, Hanssen H, Schuster T, Halle M, Koehler F. Association of physical activity and prognostic parameters in elderly patients with heart failure. J Aging Phys Act. 2011 Jan;19(1):1–15. doi: 10.1123/japa.19.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Walker PP, Burnett A, Flavahan PW, Calverley PM. Lower limb activity and its determinants in COPD. Thorax. 2008 Aug;63(8):683–689. doi: 10.1136/thx.2007.087130. [DOI] [PubMed] [Google Scholar]
- 14.Tinetti ME, McAvay GJ, Chang SS, et al. Contribution of multiple chronic conditions to universal health outcomes. J Am Geriatr Soc. 2012 Sep;59(9):1686–1691. doi: 10.1111/j.1532-5415.2011.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougherty CM, Glenny RW, Kudenchuk PJ, Malinick TE, Flo GL. Testing an exercise intervention to improve aerobic conditioning and autonomic function after an implantable cardioverter defibrillator (ICD) Pacing Clin Electrophysiol. 2010 Aug;33(8):973–980. doi: 10.1111/j.1540-8159.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty CM, Steele BG, Hunziker J. Testing an intervention to improve functional capability in advanced cardiopulmonary illness. J Cardiopulm Rehabil Prev. 2011 Jan-Feb;31(1):35–41. doi: 10.1097/HCR.0b013e3181f1fd77. [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002 Mar 21;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 18.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005 Jan 20;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 19.ATS. ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Balke B, Ware R. An experimental study of physical fitness of air force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 21.Cavanaugh JT, Coleman KL, Gaines JM, Laing L, Morey MC. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. J Am Geriatr Soc. 2007 Jan;55(1):120–124. doi: 10.1111/j.1532-5415.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- 22.Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg. 2007 Dec;46(6):1208–1214. doi: 10.1016/j.jvs.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 24.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005 Nov;37(11 Suppl):S531–543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen HQ, Burr RL, Gill DP, Coleman K. Validation of the Stepwatch device for measurement of free-living ambulatory activity in patients with COPD. Journal of Nursing Measurement. doi: 10.1891/1061-3749.19.2.76. in press. [DOI] [PubMed] [Google Scholar]
- 26.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Tudor-Locke CE, Myers AM. Methodological considerations for researchers and practitioners using pedometers to measure physical (ambulatory) activity. Res Q Exerc Sport. 2001 Mar;72(1):1–12. doi: 10.1080/02701367.2001.10608926. [DOI] [PubMed] [Google Scholar]
- 28.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Accelerometer-determined steps per day in US adults. Med Sci Sports Exerc. 2009 Jul;41(7):1384–1391. doi: 10.1249/MSS.0b013e318199885c. [DOI] [PubMed] [Google Scholar]
- 29.Tudor-Locke C, Hart TL, Washington TL. Expected values for pedometer-determined physical activity in older populations. Int J Behav Nutr Phys Act. 2009;6:59. doi: 10.1186/1479-5868-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005 May 1;171(9):972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 31.Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008 Apr 1;177(7):743–751. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- 32.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009 Feb;33(2):262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 33.Tudor-Locke C, Washington TL, Hart TL. Expected values for steps/day in special populations. Prev Med. 2009 Aug;49(1):3–11. doi: 10.1016/j.ypmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Hill K, Dolmage TE, Woon L, Coutts D, Goldstein R, Brooks D. Defining the Relationship Between Average Daily Energy Expenditure and Field-Based Walking Tests and Aerobic Reserve in COPD. Chest. 2012 Feb;141(2):406–412. doi: 10.1378/chest.11-0298. [DOI] [PubMed] [Google Scholar]
- 35.Vaes AW, Wouters EF, Franssen FM, et al. Task-related oxygen uptake during domestic activities of daily life in patients with COPD and healthy elderly subjects. Chest. 2011 Oct;140(4):970–979. doi: 10.1378/chest.10-3005. [DOI] [PubMed] [Google Scholar]
- 36.Steele B, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner DM. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000 May;117(5):1359–1367. doi: 10.1378/chest.117.5.1359. [DOI] [PubMed] [Google Scholar]
- 37.Jehn M, Schmidt-Trucksass A, Schuster T, et al. Daily walking performance as an independent predictor of advanced heart failure: Prediction of exercise capacity in chronic heart failure. Am Heart J. 2009 Feb;157(2):292–298. doi: 10.1016/j.ahj.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Sewell L, Singh SJ, Williams JE, Morgan MD. Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J Cardiopulm Rehabil Prev. 2010 Sep-Oct;30(5):329–333. doi: 10.1097/HCR.0b013e3181e175f2. [DOI] [PubMed] [Google Scholar]