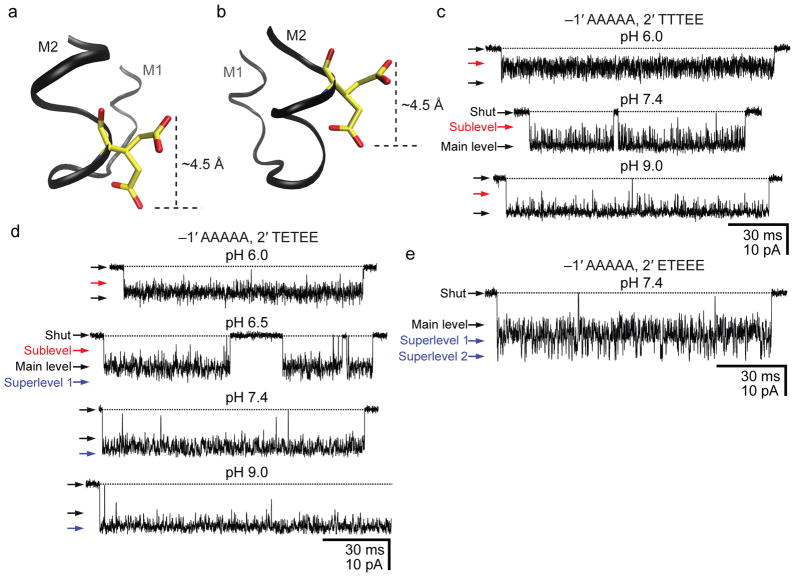
Figure 5. Moving the intermediate ring of glutamates into the pore.
a, b, Two rotamers of glutamate. Using a model of the bacterial nicotinic-receptor-like GLIC channel (pdb code: 3EAM; ref. 28), a glutamate was “engineered” at position –1′ (a) or 2′ (b) using Coot37 and VMD38 molecular-graphics software (in GLIC, the wild-type glutamates of the intermediate ring occur at position –2′). The two conformers were chosen arbitrarily from the library of Lovell and coworkers39 (see also Supplementary Fig. 5) and are merely meant to illustrate two extreme positions that the Oε1/Oε2 atoms could adopt. In going from position –1′ (a) to 2′ (b), it seems as though the glutamate would lose the ability to alternately “expose” and “hide” the negative charge to and from the pore; the charge would always be inside the pore. c, d, e, Single-channel inward currents (cell-attached configuration; ~−100 mV; 1 μM ACh; solutions 1 and 2) recorded from mutants containing the indicated residues at positions –1′ and 2′ (the latter, also in the order: α1, ε, α1, β1 and δ subunits). The indicated pH values are those of the pipette solution. The three constructs also carried theεT264P mutation. The current-amplitude scale is the same for all three panels. Openings are downward deflections. Display fc ≅ 6 kHz. We could not record currents from the mutant containing a full ring of alanines at position –1′ and a full ring of glutamates at position 2′ (~100 successful gigaohm seals with no channel activity).