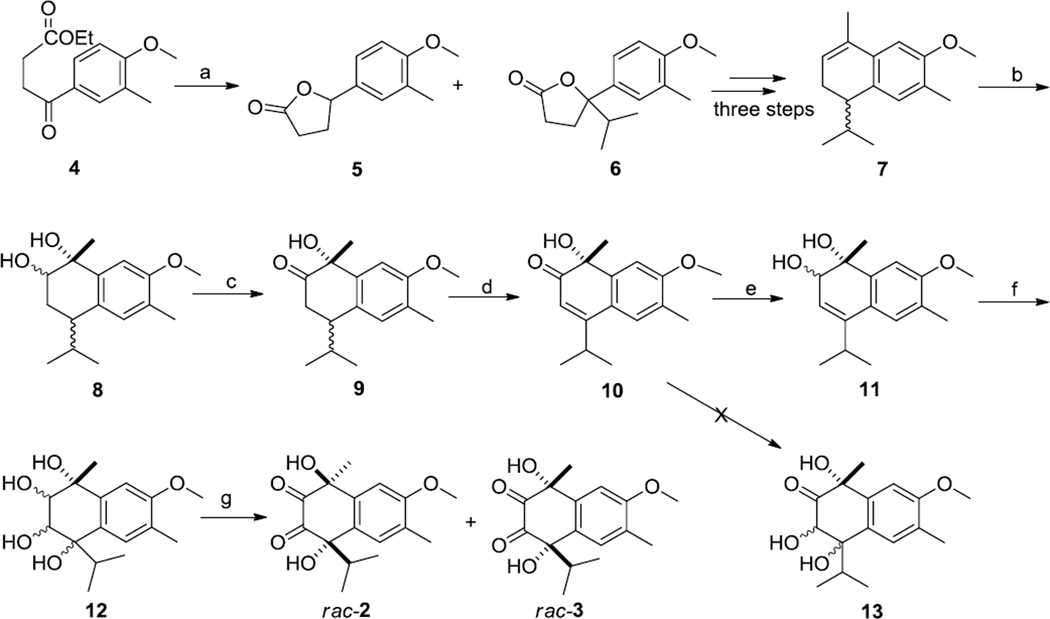
Scheme 1.
Conditions: a. Isopropylmagnesium chloride, THF, −15 °C, 1h, 42.1%; b. 2% Osmium tetroxide, N-Methyl-morpholine-N-oxide (NMO), dichloromethane, RT, 48h, 75.4%; c. Sulfur trioxide-pyridine complex, dichloromethane, 0 °C, 2h, 91.9%; d. 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), benzene, reflux, 24h, 58%; e. NaBH4, CeCl3.7H2O, rt, 5min, 90.3%. f. 2% Osmium tetroxide, N-Methyl-morpholine-N-oxide (NMO), dichloromethane, RT, 48 h; g. Sulfur trioxide-pyridine complex, dichloromethane, 0°C, 2 h, 13% (rac-2) 10% (rac-3).
Synthesis of compound 2 and 3