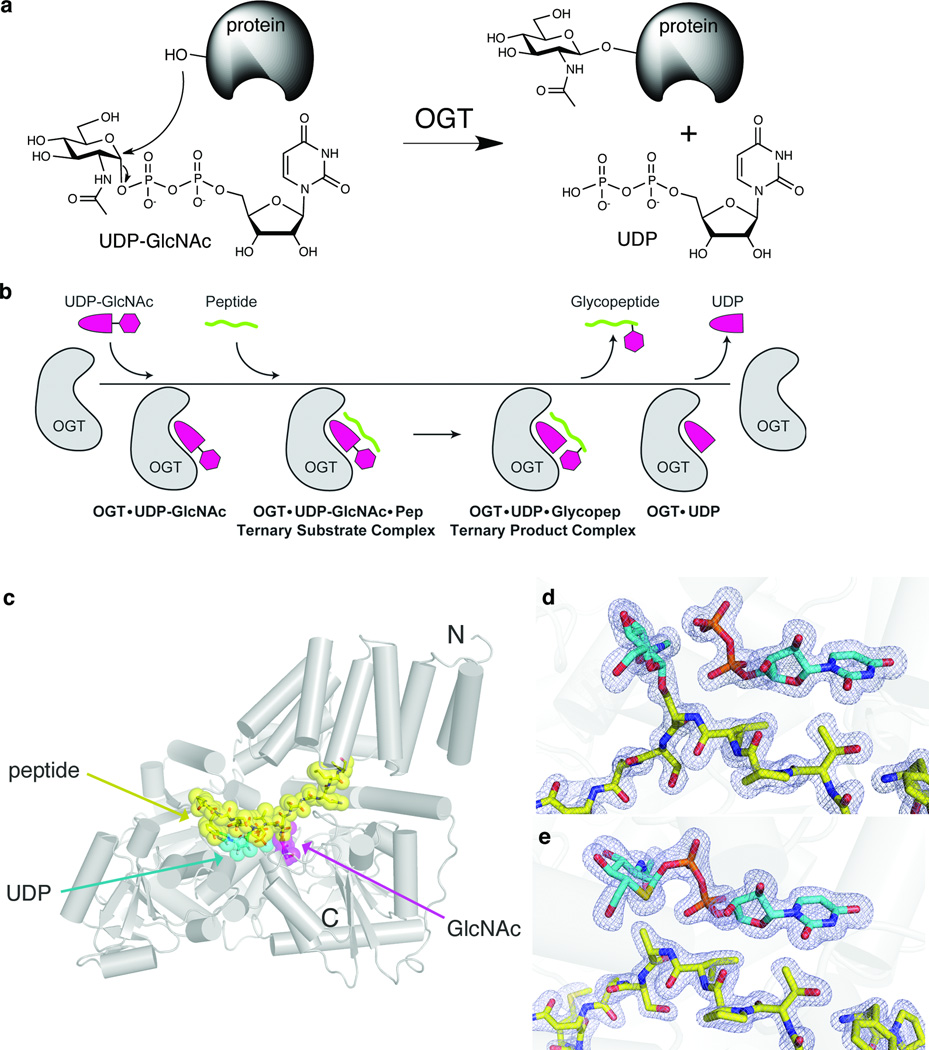
Figure 1. Structural snapshots define the kinetic mechanism of OGT.
(a) OGT catalyzes transfer of GlcNAc from UDP-GlcNAc to serine or threonine residues of nucleocytoplasmic proteins. (b) Schematic of the ordered bi-bi kinetic mechanism of OGT and the complexes obtained along the trajectory. The final binary complex, OGT-UDP, was previously obtained8. (c) Crystal structure of the ternary product complex of human OGT4.5, shown as a cartoon representation. The CKII peptide is shown in yellow, the UDP in cyan, and the GlcNAc moiety of the glycopeptide is shown in magenta. The N and C termini of the protein are indicated. (d) Electron density of the ternary product complex with the fo-fc map, shown at 3σ. The GlcNAc and the UDP are shown in cyan, and the peptide is shown in yellow. (e) Electron density of the ternary substrate complex. The fo-fc map, shown at 3σ, clearly indicates that the density connects the sugar to the UDP. The UDP-5SGlcNAc is shown in cyan, with the sulfur atom in the sugar shown in yellow.