Abstract
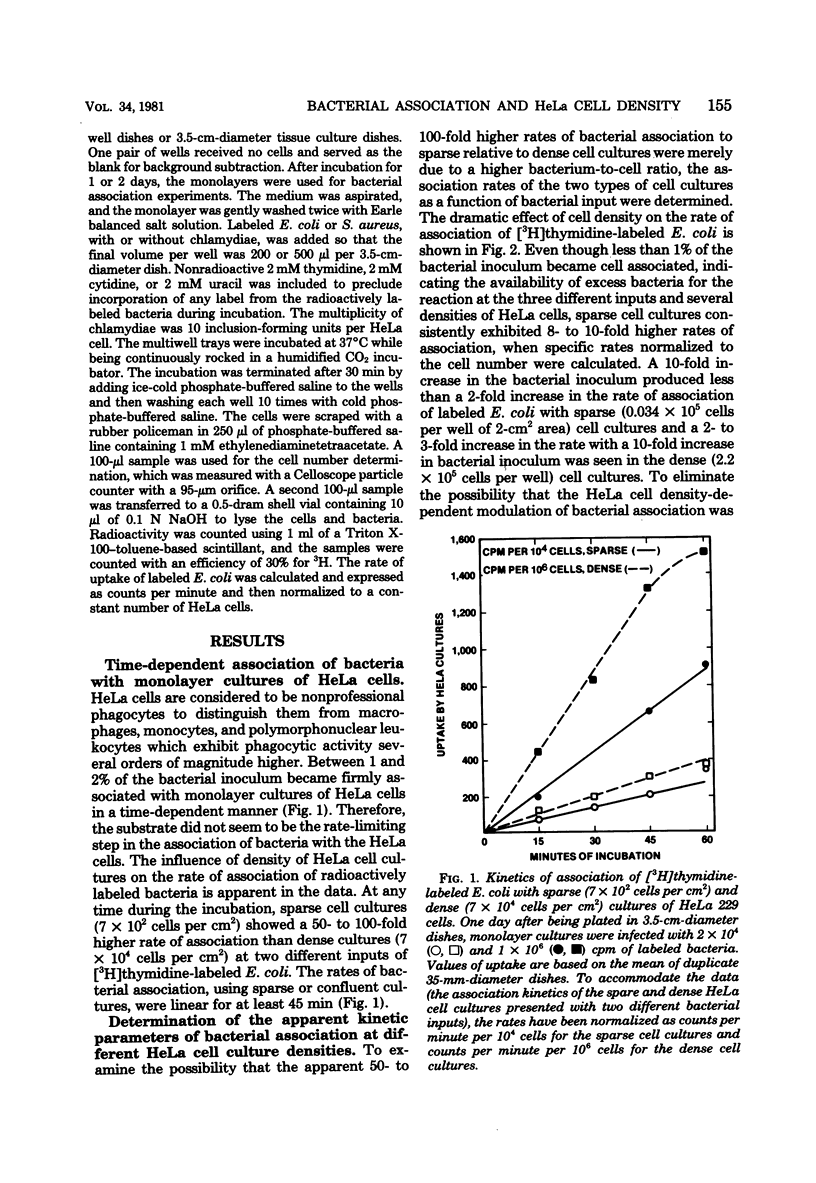
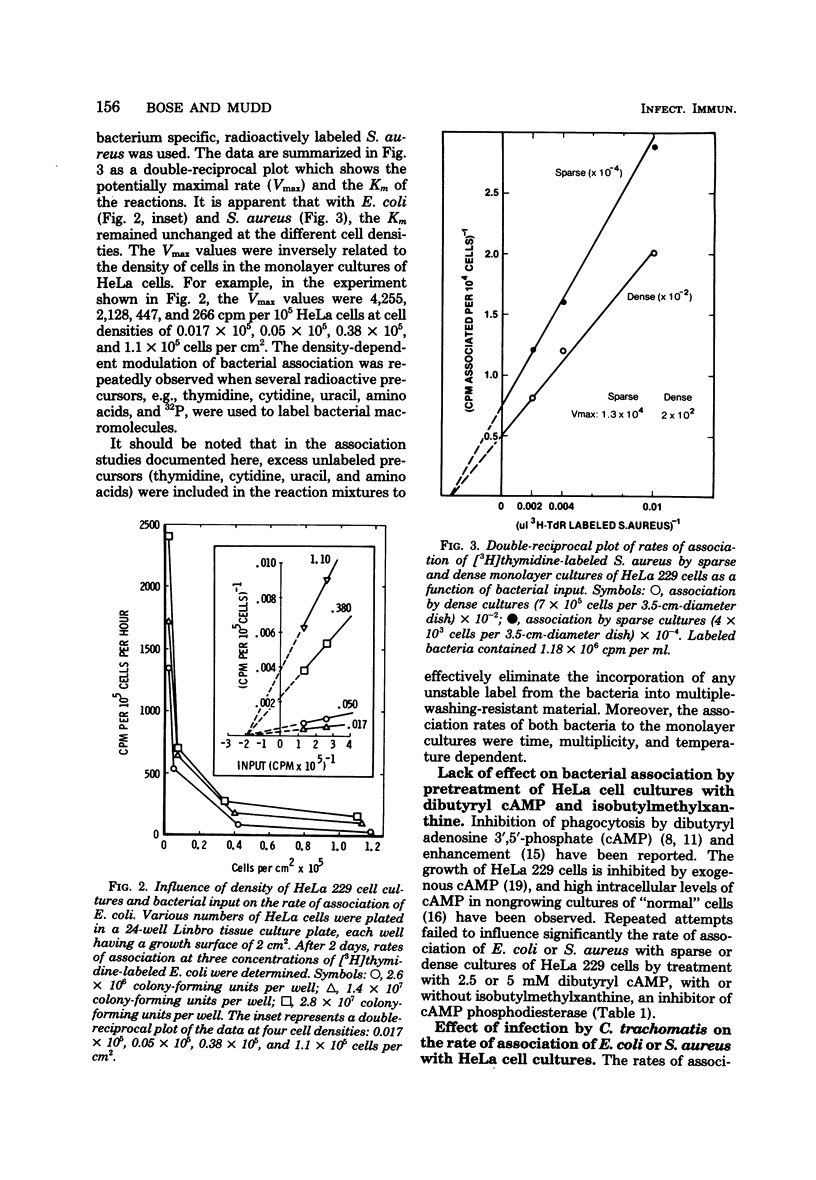
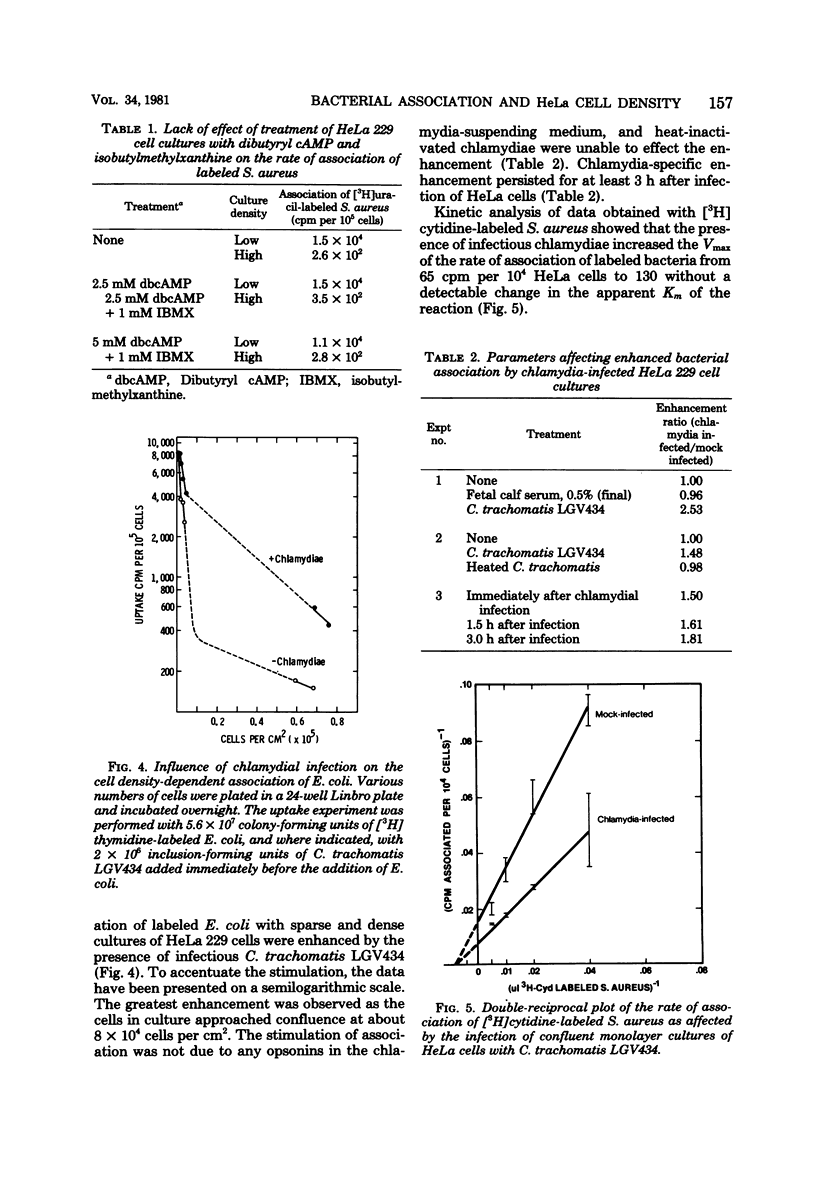
The effect of cell density on the rate of association of Escherichia coli and Staphylococcus aureus by monolayer cultures of HeLa 229 cells was investigated. Radioactively labeled bacteria were incubated with sparsely and densely plated cells. The rate of bacterial uptake was 10- to 20-fold higher in sparse cultures. Kinetic analysis of data with different multiplicities of input bacteria showed that the Km of the reaction was unaltered, whereas the Vmax was inversely related to cell density. Pretreatment of HeLa cultures with dibutyryl adenosine 3',5'-phosphate had little effect on the rate of bacterial association. The simultaneous presence of an obligately parasitic bacterium, Chlamydia trachomatis LGV434, enhanced the Vmax of association of E. coli and S. aureus. This effect was more pronounced in dense HeLa cell cultures. Heat-inactivated chlamydiae were unable to modify the association. Enhanced association persisted for at least 3 h after infection with chlamydiae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose S. K., Liebhaber H. Deoxyribonucleic acid synthesis, cell cycle progression, and division of Chlamydia-infected HeLa 229 cells. Infect Immun. 1979 Jun;24(3):953–957. doi: 10.1128/iai.24.3.953-957.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S. K., Warren R. J. On the stability of phage messenger RNA. Biochem Biophys Res Commun. 1967 Feb 21;26(4):385–391. doi: 10.1016/0006-291x(67)90557-8. [DOI] [PubMed] [Google Scholar]
- Bose S. K., Zlotnick B. J. Growth-and density-dependent inhibition of deoxyglucose transport in Balb 3T3 cells and its absence in cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2374–2378. doi: 10.1073/pnas.70.8.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978 Feb;19(2):607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med. 1960 May 1;111:667–687. doi: 10.1084/jem.111.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. P., Karnovsky M. L. The depression of phagocytosis by exogenous cyclic nucleotides, prostaglandins, and theophylline. J Cell Biol. 1973 Nov;59(2 Pt 1):480–490. doi: 10.1083/jcb.59.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory S. H., Bose S. K. Density-dependent changes in hexose transport, glycolytic enzyme levels, and glycolytic rates, in uninfected and murine sarcoma virus-transformed rat kidney cells. Exp Cell Res. 1977 Dec;110(2):387–397. doi: 10.1016/0014-4827(77)90305-6. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Silverstein S. C. Segmental response of the macrophage plasma membrane to a phagocytic stimulus. J Exp Med. 1974 Feb 1;139(2):323–336. doi: 10.1084/jem.139.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. Cell contact induces an increase in pinocytotic rate in cultured epithelial cells. Nature. 1976 Oct 14;263(5578):596–597. doi: 10.1038/263596a0. [DOI] [PubMed] [Google Scholar]
- Kruth H. S., Avigan J., Gamble W., Vaughan M. Effect of cell density on binding and uptake of low density lipoprotein by human fibroblasts. J Cell Biol. 1979 Dec;83(3):588–594. doi: 10.1083/jcb.83.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel R. J., Rosen N., Rosen O. M., Bloom B. R. Modulation of Fc-mediated phagocytosis by cyclic AMP and insulin in a macrophage-like cell line. J Immunol. 1977 Nov;119(5):1813–1820. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L. Kinetics of phagocytosis of Staphylococcus aureus by alveolar and peritoneal macrophages. Infect Immun. 1979 Nov;26(2):479–486. doi: 10.1128/iai.26.2.479-486.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan W. L., Heidrick M. L. Inhibition of cell growth in vitro by adenosine 3',5'-monophosphate. Science. 1968 Dec 27;162(3861):1484–1485. doi: 10.1126/science.162.3861.1484. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Fielding P. E., Fielding C. J., Gospodarowicz D. Role of contact inhibition in the regulation of receptor-mediated uptake of low density lipoprotein in cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):356–360. doi: 10.1073/pnas.75.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]


