Abstract
Objective
To investigate the presence of primary and secondary hyperalgesia among subjects with chronic subacromial impingement syndrome (SIS) compared to pain-free controls.
Design
Cross-sectional design.
Setting
Outpatient rehabilitation clinic, urban, academic medical center.
Participants
Volunteer sample of 62 subjects (31 with SIS, 31 controls).
Interventions
Not applicable.
Main Outcome Measures
Pressure-pain thresholds (PPTs) were measured at the middle deltoid of the affected/dominant arm (primary or secondary hyperalgesia) and the middle deltoid and tibialis anterior of the unaffected/non-dominant side (secondary hyperalgesia) in SIS and healthy controls, respectively. Differences in PPTs were analyzed by Wilcoxon Rank Sum test and with linear regression analysis controlling for gender, a known confounder of PPTs.
Results
After adjusting for gender, subjects with SIS had significantly lower PPTs than controls at all locations. Controls had a 1.4 kg/cm2 (95% CI 1.2 – 1.5) higher PPT at their affected shoulder than those with SIS, a 0.7 kg/cm2 (95% CI 0.5 – 0.9) higher PPT at their non-affected shoulder, and a 1.1 kg/cm2 (95% CI 1.1 – 1.2) higher PPT at their contralateral tibialis anterior. Observers were not blinded to patient groupings but were blinded to level of applied pressure.
Conclusion
This study provides further evidence that SIS patients have significantly lower PPTs than controls in both local and distal areas from their affected arm consistent with primary and secondary hyperalgesia, respectively. Data suggest the presence of central sensitization among subjects with chronic SIS.
Keywords: Hypersensitivity, Pain Thresholds, Subacromial Impingement Syndrome
Subacromial impingement syndrome (SIS) is a common cause of shoulder pain, estimated to be the cause for up to half of incident cases.1 Anatomically, SIS refers to the supraspinatus tendon impinging on the undersurface of the anterior acromion as the arm is raised overhead.2 Typically, pain is generated with elevation of the arm above the head though it can occur with rest.3 Many pathologies are found in those with SIS, either alone or conjointly, and include subacromial bursitis, rotator cuff tendinopathy, and partial rotator cuff tears.4 Treatment generally consists of conservative therapy, though as duration of shoulder pain from SIS increases, the likelihood of successful treatment becomes worse.5 Up to 45% of those who present to their primary physicians with shoulder pain will either continue to have pain at 2 years or will have gone on to surgical treatment.6 Unfortunately, randomized controlled trials have not shown the benefit of surgical treatment over conservative treatment,7,8 leaving a large number of those with shoulder pain to suffer from chronic pain. There is now evidence that alterations in the central and peripheral nervous systems may play a role in chronic pain,9,10,11 and may explain why some patients fail to improve in spite of treatment and lack of evidence for persistent pathology.
There is evidence of secondary hyperalgesia in those who experience chronic shoulder pain from SIS, providing indirect evidence of central hypersensitivity.12 Central hypersensitivity is an augmentation of the nociceptive pathways of the central nervous system9,10,11 that is characterized by local and generalized lowered pain thresholds and an exaggerated pain response to painful and non-painful stimulation. Central hypersensitivity is a normal response of the central nervous system to injury that encourages protection of injured tissue to allow healing.13 After the injured tissue has healed, the hypersensitivity to pain typically resolves; however, the central hypersensitivity may persist in some individuals, resulting in a chronic pain syndrome. In the case of those with SIS, central hypersensitivity may be associated with persistent pain in spite of treatment.
Local and generalized hyperalgesia was demonstrated by Hidalgo-Lozano, et al. in the form of lower pain thresholds (i.e. pain being perceived at lower mechanical pressure intensities) in the deltoid (local) and ipsilateral tibialis anterior (distal, healthy tissue) compared to control subjects.12 Hyperalgesia in the shoulder of those with SIS compared to controls could represent either primary or secondary hyperalgesia, which are indirect evidence of peripheral and central hypersensitivity, respectively. Hyperalgesia in distal, healthy tissues in those with SIS compared to controls is evidence of central hyperalgesia as a lower pain threshold in uninvolved tissue would require alterations in the central nervous system.10 As noted by the authors, a major limitation of the study was a small sample size. Other limitations in this study include lack of adjusting for gender, age, and ethnicity, all of which have been shown to independently influence pain thresholds,14 –17 lack of evaluating pain thresholds contralateral to the affected side, and not clearly excluding all subjects with other chronic pain syndromes. The objective of this study was to address these limitations and to confirm the presence of primary and secondary hyperalgesia among subjects with chronic shoulder pain due to SIS compared to a pain-free population.
Methods
Subjects
This cross-sectional study was approved by the institutional review board of the authors’ local institution. Subjects were recruited from an outpatient rehabilitation clinic including physician and allied health services of an urban, academic medical center. After obtaining informed consent and establishing eligibility, baseline information was collected. Subjects with SIS were 21 years or older, had shoulder pain for at least six months, and had shoulder pain of 4 or greater in the last week on a scale 0 to 10. Subjects with SIS were excluded if they had evidence of joint or overlying skin infection, prior surgery to the affected limb, or had any other chronic pain syndrome. Controls were 21 years or older, were without pain in the prior week greater than 3 on scale 0 to 10, and had no pain in a single location for more than 16 days of the last 30. Subjects were also excluded if they had evidence of joint or overlying skin infection or had difficulty understanding instructions related to determination of a pain threshold.
Pressure-pain threshold
Hyperalgesia is evaluated by measuring pressure-pain thresholds (PPT), the minimum amount of pressure at which pain is perceived.18 The reliability of pressure algometry to evaluate deep somatic tissue sensitivity has been demonstrated previously.19,–,21 To discriminate between peripheral and central sensitization, sites of healthy tissue, distal to the site of injury were included. Secondary hyperalgesia, a reduction in pain thresholds in healthy tissue distal to the site of injury in those with SIS compared to controls is indicative that a central process is responsible.10
Two assessors obtained PPT measurements in subjects with HSP and controls. The assessors underwent training prior to the study to standardize measurement methods including subject positioning, assessor blinding to pressure reading, and rate of pressure application. Pressure-pain thresholds were measured using a hand-held digital algometera with 1cm2 rubber tip by applying the probe perpendicularly to the skin at a rate of 1 kg/cm2 per second while subjects were seated comfortably with their arms at their sides. The mean of 3 trials at each location were calculated and used for analysis. The PPTs were measured at the middle deltoid of the affected/dominant arm, the unaffected/non-dominant arm, and the tibialis anterior on the same side of the body as the unaffected/non-dominant arm in those with SIS and controls, respectively. The measurements were obtained in the same order on all subjects.
Statistical Analysis
Differences in demographic variables between the two groups were analyzed with a chi-square test, or Fisher’s exact tests for small cell size (< 5) for categorical variables and with the Wilcoxon Rank-Sum test for continuous variables. The effect sizes of PPTs between SIS and controls were calculated by the differences of the predicted PPTs from linear regression models with the covariates of gender and age, both known to influence PPTs.14,15 The covariate ethnicity16,17 was also evaluated in the prediction models because of its association with PPTs, though could not be included in the models due to insufficient distribution across groups. Confidence intervals were calculated by repeating the regression analysis on boot-strapped samples (with replacement, 1000 iterations) and finding the 2.5 and 97.5 percentiles of the estimates. Due to non-normal distributions of the PPTs, we transformed the measurements in the primary and subgroup analyses, respectively, of the local deltoid (fourth root, square root), non-affected deltoid (square root, square root) and tibialis anterior (square root, no transformation) prior to the regression analyses. The predicted values were back-transformed to the original scale before calculation of PPT differences.
Hypothesis
We hypothesized that subjects with SIS will have significantly lower pain thresholds local to and distal from their painful shoulders than healthy control subjects.
Results
A total of 64 subjects were enrolled in this study, of which 2 were excluded for difficulty understanding instructions related to the measurement of PPTs. There were 31 cases and 31 controls in the analysis. There were significant differences between groups with those with SIS being older and having a larger minority representation, as can be seen in Table 1.
Table 1.
Demographic Information
| SIS Patients | Controls | ||
|---|---|---|---|
| n | 31 | 31 | |
| Female (%) | 16 (51.6) | 21 (67.7) | p=0.2 |
| Age,years(std) Ethnicity | 51.7 (10.0) | 39.5 (10.9) | p<0.02 |
| Caucasian n,(%) | 16 (51.6) | 29 (93.6) | p<0.001 |
| African-American n,(%) | 12 (38.7) | 1 (3.2) | p=0.001 |
| Other n,(%) | 3 (9.7) | 1 (3.2) | p=0.6 |
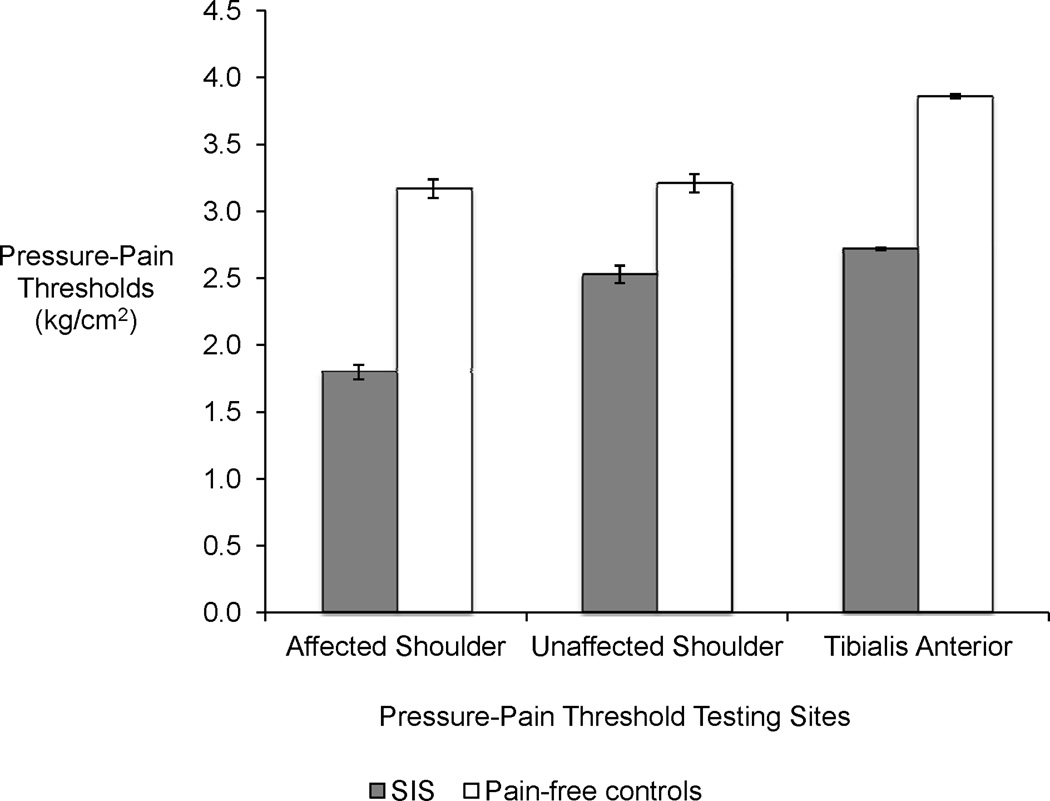
The mean PPTs for SIS and controls at their affected/dominant shoulder and contralateral shoulder and tibialis anterior are shown in Figure 1. We used linear regression models to predict the effect size of the differences in PPTs for subjects with SIS compared to controls when taking into account gender and age differences. The models revealed significantly lower PPTs for subjects with SIS at all locations tested. The estimated effect sizes for PPTs for those with SIS were lower than controls at their affected shoulder by 1.4 kg/cm2 (95% CI 1.2 – 1.5), lower by 0.7 kg/cm2 (95% CI 0.5 – 0.9) at their non-affected shoulder, and lower by 1.1 kg/cm2 (95% CI 1.1 – 1.2) at their contralateral tibialis anterior.
Figure 1.
Pressure-Pain Thresholds (kg/cm2) of subjects with SIS (n=31) and pain-free controls (n=31). The errors bars indicate standard error.
To control for potential differences in race that we could not adjust for in our models, the analyses were repeated on Caucasians as they represented 73.4% of the sample. The estimated effect sizes for PPTs for those with SIS were lower than controls at their affected shoulder by 1.0 kg/cm2 (95% CI 0.7 – 1.4), lower by 0.6 kg/cm2 (95% CI 0.3 – 0.9) at their non-affected shoulder, and lower by 0.8 kg/cm2 (95% CI 0.6 – 0.9) at their contralateral tibialis anterior.
Discussion
The current results are in agreement with prior work that demonstrated lower PPT’s in local and ipsilateral distal locations in patients with chronic SIS, consistent with primary and secondary hyperalgesia, respectively.12 Our study provides greater evidence for widespread central hypersensitivity through lower PPTs on the contralateral shoulder and contralateral tibialis anterior in addition to the affected shoulder in SIS subjects compared to control subjects. We also improved on the prior study by recruiting a larger sample (62 subjects compared to 22), by excluding all subjects with a chronic pain syndrome other than SIS, and by adjusting for the effect of gender in our analyses.
Reduced pain thresholds at healthy tissues distal to the affected shoulder provide further support for the presence of widespread central hypersensitivity for those with chronic SIS. Lower pain thresholds compared to normal values in pain-free control subjects has been suggested as a clinically meaningful difference.27 Persistence of central hypersensitivity has been suggested in multiple chronic pain conditions, demonstrated by reduced pain thresholds in healthy tissues distal to the site of injury when compared to healthy control subjects.11,12,22–26 The association of chronic pain and central hypersensitivity is not well understood, though there is evidence that persistent central hypersensitivity is a consequence of chronic pain rather than a risk factor for chronic pain.28 This finding raises the possibility that successfully treating chronic SIS may need to address more than biomechanical or anatomic pathologies alone.
Study Limitations
There are limitations to our study that should be kept in mind. We were unable to limit analgesic usage among the subjects with SIS. However, medication use would be expected to raise the PPTs for those with SIS and bias against a difference between the PPT’s of subjects with SIS controls. Differences in estimated PPTs were found despite this limitation, though it may cause underestimation of the true difference in PPTs between those with chronic pain and without. Second, our sample was not a random sample; thus the true difference in PPTs in those with chronic SIS and without may be different than that seen in this study. Third, the evaluators were not blinded to case and control subjects and could have potentially introduced bias into the study; however, efforts were made to reduce bias by blinding evaluators to PPT levels during testing. Finally, there were significant differences in gender, age and ethnicity that could contribute to differences between those with SIS and controls. While we adjusted for gender and age, we could not adjust for race within the models due to the lack of distributional overlap between the groups. Race has been shown to be significantly associated with pain-perception,16,17 with minorities having lower-pain thresholds than Caucasians, and this could bias towards the differences observed in this study. We repeated the analyses in Caucasians only and, while the magnitude of difference is lower, widespread hyperalgesia is observed in those subjects with SIS compared to pain-free controls and thus the conclusions of our study are unchanged.
Conclusions
This study provides further evidence that subjects with chronic shoulder pain due to SIS have lower pain thresholds than control subjects in both local areas and distal areas from their affected arm. This suggests that subjects with SIS may be experiencing central hypersensitivity. Further studies of the relationship of PPTs and chronic pain syndromes should be conducted including longitudinal studies of central hypersensitivity in subjects with chronic shoulder pain undergoing treatment which would improve our understanding of this association.
Acknowledgement
Steven M. Sidik, affiliated with Cleveland FES Center (Staff Statistician) and Department of Statistics, Case Western Reserve University (Lecturer) for assistance in statistical analyses.
Funding: Tracy Paul, received scholarship funds from Case Western Reserve University School of Medicine for this study. Material support and salary support (Chae, Wilson) were provided through K24HD054600 from NICHD/NIH. Secure data storage was made possible through grants M01 RR00080 and UL1 RR024989 from NCRR/NIH. John Chae is a consultant and Chief Medical Advisor to SPR Therapeutics, a medical device company in Cleveland, OH. Dr. Chae also owns equity in SPR Therapeutics. Although SPR Therapeutics did not fund this study, the company has a vested interest in the study content.
Abbreviations
- PPT
Pressure-Pain Threshold
- SIS
Subacromial Impingement Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was in part presented at the Annual Meeting of the Association of Academic Physiatrists, Las Vegas, NV, March, 2012.
Financial Disclosure:
A commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer a financial benefit on the author or 1 or more of the authors. John Chae MD is a consultant and Chief Medical Advisor to SPR Therapeutics, a medical device company in Cleveland, OH. Dr. Chae also owns equity in SPR Therapeutics. Although SPR Therapeutics did not fund this study, the company has a vested interest in the study content.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated. (Paul, Soo Hoo, Wilson)
Suppliers’ List:
Hand-held digital algometer (Wagner Pain Test – Model FPIX Digital Algometer, Wagner Instruments, Post Office Box 1217, Greenwich, CT, 06836-1217 USA)
References
- 1.van der Windt DAWM, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54:959–964. doi: 10.1136/ard.54.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neer CS., II Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54:41–50. [PubMed] [Google Scholar]
- 3.Neer CS., II Impingement lesions. Clin Orthop Relat Res. 1983;173:70–77. [PubMed] [Google Scholar]
- 4.Bigliani LU, Levine WN. Subacromial impingement syndrome. J Bone Joint Surg Am. 1997;79(12):1854–1868. [PubMed] [Google Scholar]
- 5.Kuijpers T, van der Windt DAWM, Boeke AJP, Twisk JWR, Vergouwe Y, Bouter LM, van der Heijden GJMG. Clinical prediction rules for the prognosis of shoulder pain in general practice. Pain. 2006;120:276–285. doi: 10.1016/j.pain.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of non-operative treatment. J Shoulder Elbow Surg. 2009;18:172–177. doi: 10.1016/j.jse.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Dorrestijn O, Stevens M, Winters JC, van der Meer K, Diercks RL. Conservative or surgical treatment for subacromial impingement syndrome? A systematic review. J Shoulder Elbow Surg. 2009;18:652–660. doi: 10.1016/j.jse.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Ketola S, Lehtinen J, Arnala I, Nissinen M, Westenius H, Sintonen H, Aronen P, Konttinen YT, Malmivaara A, Rousi T. Does arthroscopic acromioplasty provide any additional value in the treatment of shoulder impingement syndrome? a two-year randomised controlled trial. J Bone Joint Surg Br. 2009;91(10):1326–1334. doi: 10.1302/0301-620X.91B10.22094. [DOI] [PubMed] [Google Scholar]
- 9.Petersen-Felix S, Curatolo M. Neuroplasticity – an important factor in acute and chronic pain. Swiss Med Wkly. 2002;132:273–278. doi: 10.4414/smw.2002.09913. [DOI] [PubMed] [Google Scholar]
- 10.Curatolo M, Arendt-Nielsen L, Petersen-Felix S. Central hypersensitivity in chronic pain: mechanisms and clinical implications. Phys Med Rehabil Clin N Am. 2006;17:287–302. doi: 10.1016/j.pmr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009 Sep;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidalgo-Lozano A, Fernández-de-las-Peñas C, Alonso-Blanco C, Ge HY, Arendt- Nielsen L, Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Exp Brain Res. 2010;202(4):915–925. doi: 10.1007/s00221-010-2196-4. [DOI] [PubMed] [Google Scholar]
- 13.Greene CS. Neuroplasticity and sensitization. JADA. 2009;140:676–678. doi: 10.14219/jada.archive.2009.0253. [DOI] [PubMed] [Google Scholar]
- 14.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. Gender differences in pressure pain threshold in healthy humans. Pain. 2003;101:259–266. doi: 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 15.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic Differences in Pain Tolerance: Clinical Implications in a Chronic Pain Population. Psychosomatic Medicine. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Mechlin BM, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans Show Alterations in Endogenous Pain Regulatory Mechanisms and Reduced Pain Tolerance to Experimental Pain Procedures. Psychosomatic Medicine. 2005;67:948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- 18.Vanderweeën L, Oostendorp RA, Vaes P, Duquet W. Pressure algometry in manual therapy. Man Ther. 1996;1(5):258–265. doi: 10.1054/math.1996.0276. [DOI] [PubMed] [Google Scholar]
- 19.Nussbaum EL, Downes L. Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Phys Ther. 1998;78(2):160–169. doi: 10.1093/ptj/78.2.160. [DOI] [PubMed] [Google Scholar]
- 20.Fischer AA, editor. Muscle pain syndromes and fibromyalgia Pressure algometry for quantification of diagnosis and treatment outcome. New York: Haworth Medical Press; 1998. pp. 1–158. [Google Scholar]
- 21.Chesterton LS, Sim J, Wright CC, Foster NE. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin J Pain. 2007;23(9):760. doi: 10.1097/AJP.0b013e318154b6ae. [DOI] [PubMed] [Google Scholar]
- 22.Scott D, BPhyty (Hons), Jull G, Sterling M. Widespread sensory hypersensitivity is a feature of chronic whiplash-associated disorder but not chronic idiopathic neck pain. Clin J Pain. 2005;21:175–181. doi: 10.1097/00002508-200503000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis and Rheumatism. 2003;48(5):1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-de-las-Peñas C, de la Llave-Rincón AI, Fernández-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain. 2009;132(6):1472–1479. doi: 10.1093/brain/awp050. [DOI] [PubMed] [Google Scholar]
- 25.Bendtsen L, Jensen R, Olesen J. Decreased pain detection and tolerance thresholds in chronic tension-type headache. Arch Neurol. 1996;53:373–376. doi: 10.1001/archneur.1996.00550040113021. [DOI] [PubMed] [Google Scholar]
- 26.Svensson P, List T, Hector G. Analysis of stimulus-evoked pain in patients with myofascial temporomandibular pain disorders. Pain. 2001;92:399–409. doi: 10.1016/S0304-3959(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 27.Fischer AA. Pressure Threshold Meter: Its Use for Quantification of Tender Spots. Arch Phys Med Rehabil. 1986;67:839–838. [PubMed] [Google Scholar]
- 28.Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R. Increased pain sensitivity is not a risk factor but a consequence of frequent headache: a population-based follow-up study. Pain. 2008;137:623–630. doi: 10.1016/j.pain.2007.10.023. [DOI] [PubMed] [Google Scholar]