Abstract
In this study, we have characterized the immune mechanisms elicited by antigenic candidates, TcG2 and TcG4, delivered by a DNA-prime/MVA-boost approach, and evaluated the host responses to T. cruzi infection in C57BL/6 mice. Immunization of mice with antigenic candidates elicited antigen-specific, high-avidity, trypanolytic antibody response (IgG2b>IgG1) and CD8+T cells that exhibited type-1 cytolytic effector (CD8+CD107a+IFN-γ+Perforin+) phenotype. The extent of TcG2-dependent type 1 B and T cell immunity was higher than that noted in TcG4-immunized mice, and expanded accordingly in response to challenge infection with T. cruzi. The progression of chronic phase in immunized mice was associated with persistence of IgGs, 55–90% reduction in the frequency of proinflammatory (IFN-γ+ or TNF-α+) CD8+T cells, and an increase or emergence of immunoregulatory (IL-10+) CD4/CD8 T cells. The tissue parasitism, infiltration of inflammatory infiltrate, parasite persistence, and fibrosis were decreased by 82–92% in heart and skeletal muscle of immunized/chronically-infected mice. Control mice exhibited a significantly low antibody response, consistent activation of effector CD8+T cells dominated by pro-inflammatory phenotype and mixed cytokine profile (IFN-γ+TNF-α>IL-4+IL-10), parasite persistence and pathologic damage in chagasic hearts. We conclude that delivery of TcG2or TcG4by DNA-rMVA approach elicits effective antibody and CD8+T cell mediated immunity against T. cruzi and Chagas disease. The emergence of type 2 cytokine and T cell responsein chronic phase was indicative of prevention of clinical disease.
Keywords: Chagas disease, Trypanosoma cruzi, DNA-prime/MVA-boost approach, antigenic candidates, effector B and T cell responses
INTRODUCTION
The protozoan parasite Trypanosoma cruzi, transmitted by blood-sucking triatomines, causes Chagas disease, which is a health threat for an estimated 10 million people, living mostly in Latin America. The congenital, blood transfusion and organ transplantation related transmissions are becoming recognized as significant threats in recent decades [1,2].
The current literature on Chagas disease suggest that a low-grade, systemic infection with documented immune-adverse reactions contribute to tissue injury, and subsequently, to cardiac insufficiency in chronically infected patients [3,4]. It is accepted that controlling the acute parasite load below a threshold level would be effective in decreasing the tissue damage imposed by multiple pathogenic mechanisms and lead to decreased disease severity, thus, providing an impetus for vaccine development against T. cruzi. Accordingly, several antigens have been tested as vaccine candidates in eliciting immunity to T. cruzi in small animal models (reviewed in [5]). In parallel, efforts to enhance the protective efficacy of subunit vaccines against T. cruzi have included testing the use of adjuvants, e.g. saponin, cytokines [5], attenuated Salmonella [6] and adenovirus [7].
We have employed a computational/bioinformatics approach for unbiased screening of the T. cruzi genome database, and identified 11 potential candidates. Through rigorous analysis over a period of several years, we considered two candidates (TcG2, TcG4) were maximally relevant for vaccine development because these candidates were highly conserved in clinically relevant T. cruzi strains, expressed (mRNA/protein) in infective and intracellular stages of T. cruzi, and recognized by IgGs and CD8+ T cells in multiple T. cruzi-infected hosts [8,9].
Delivery of vaccine candidates as DNA vaccine has been shown to elicit T cell immunity. Clinical trials of new recombinant viral vaccine candidates for malaria, tuberculosis, or HIV/AIDS have shown unprecedented levels of efficacy in eliciting antigen-specific cellular immunity. Thus, in this study, we have tested the efficacy of TcG2 and TcG4 antigenic candidates, delivered by a DNA-prime/virus-boost approach, for their ability to induce immunity and protection against T. cruzi infection and Chagas disease. We used Modified Vaccinia Ankara (MVA) for the delivery of antigens, shown to accommodate multiple foreign genes and generate cellular and humoral responses to a variety of foreign antigens [10]. We discuss the function of candidate antigens-specific antibody and T cell responses, and their efficacy in providing resistance to T. cruzi infection and Chagas disease in mice.
MATERIALS AND METHODS
Parasites and mice
T. cruzi trypomastigotes (Sylvio X10/4) were maintained and propagated by continuous in vitro passage in C2C12 cells. C57BL/6 female mice (6-to-8-weeks old) were obtained from Harlan Labs. Animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals and approved by the UTMB Animal Care and Use Committee.
T. cruzi genes and generation of recombinant plasmids, proteins and MVA viruses
The cDNAs for TcG2 and TcG4 (Genbank: AY727915 and AY727917, respectively) were cloned in pCDNA3.1 for delivery as DNA vaccine [11] and in-frame with a C-terminal His-tag in pET-22b plasmid (Novagen) for purification of recombinant proteins by poly-histidine fusion peptide-metal chelation chromatography system [12]. For the generation of recombinant MVA clones, cDNA for TcG2 and TcG4 were sub-cloned into pLW44 at the Xma1/Sbf1 sites. The pLW44 vector consist a green fluorescent protein (GFP) and multiple cloning site cassette flanked by a pair of MVA genomic sequences for homologous recombination and incorporation of GFP and gene of interest into deletion III locus of wild-type MVA (wtMVA) genome. BHK-21 cells were cultured to 70% confluency in six-well plates, and infected with wtMVA (MOI:0.05) for one h. Cells were then transfected with recombinant pLW44/Lipofectamine-2000 (Invitrogen), and after 48h, cell lysates were utilized at 10-fold dilutions to infect new BHK-21 monolayers. The GFP+ fluorescent plaques of rMVA were purified 4–6 times to remove wtMVA contaminants, and amplified by infection of BHK-21 for 72h. Cell pellets were lysed, centrifuged to remove debri, supernatants were passed through 36% sucrose cushion twice; and purified recombinant virus were stored in 1 mM Tris-HCl (pH 9) at −80°C [13].
Immunization and challenge infection
C57BL/6 mice were injected with pCDNA3.TcG2 or pCDNA3.TcG4 (25-μg/mouse, i.m.), and corresponding rMVA (106-pfu/mouse, i.d.) at 3-weeks interval (controls: empty vector), and two-weeks after the last immunization, challenged with T. cruzi (10,000 trypomastigotes/mouse, i.p.). Mice were sacrificed at 30- and 120-days post-infection (dpi), and sera and tissue samples were stored at 4°C and −80°C, respectively.
Antibody levels, avidity and trypanolytic activity
The 96-well plates were coated with T. cruzi lysate (TcTL, 5×105 parasites’ equivalent/well) or recombinant TcG2 and TcG4 (1-μg/well), blocked with PBS/5% non-fat dry milk (NFDM, Bio-Rad), and then sequentially incubated with sera samples (100-μl/well) and biotin-conjugated goat anti-mouse IgG or IgG subtypes for 2h each, and streptavidin-HRP conjugate for 30-min (from Southern Biotech, 1:5000 dilution in PBS-0.01%Tween-20/0.5%NFDM, 100-μl/well). Colorimetric reaction was performed with TMB substrate (K&P Labs) and absorbance recorded at 450 nm [12]. ELISA was also performed in presence of 6M urea, and antibody avidity calculated as [O.D. with urea/O.D. without urea]× 100 [14]. Trypanolytic activity was measured by incubating T. cruzi trypomastigotes (5×104/25-μl) for 4h at 37°C with 25-μl each of sera samples and human complement (Sigma), and percentage of live parasites monitored by MTT assay [15].
Lymphocytes’ proliferation, cytokine response and functional characterization
Splenocytes (106-cells/ml/well, 24-well plates) were incubated with ConA (5-μg/ml), recombinant proteins (10-μg/ml), or TcTL (25-μg/ml) for 48h. Culture supernatants were used for cytokines’ measurement using optEIAtm ELISA kits (Pharmingen). Splenocytes were labeled for 30-min on ice with PE-Cy7-conjugated anti-CD3 (binds T cells), FITC-conjugated anti-CD8 and PE-conjugated anti-CD4 antibodies (0.5–1μg/100μl, e-Biosciences), fixed (2% paraformaldehyde), and analyzed on a LSRII Fortessa Cell Analyzer (BD-Biosciences). For functional characterization, splenocytes were in vitro stimulated as above, except that brefeldin A (10-μg/ml; Sigma) was added in the final 6h to prevent protein secretion. Cells were labeled for CD4/CD8, fixed, permeabilized (0.1% saponin/1% FBS), and then submitted to staining with APC-anti-IL-4, PerCPCy5.5-anti-IL-10, e-Fluor-anti-IFN-γ, Cy5-anti-TNF-α, PerCP-Cy5.5-anti-Ki67, APC-anti-perforin or Alexa-Fluor488-anti-CD107 antibodies (0.5–2-μg/100-μl, e-Biosciences). Cells stained with isotype-matched IgGs were used as controls. Flow cytometry was performed, acquiring 30–50,000 events in a live lymphocyte gate, and data analyzedusing FlowJo software (v7.6.5, Tree-Star).
Tissue parasite burden and histopathology
Total DNA (50-ng) was isolated from skeletal muscle and heart tissues [16], and used as a template in real-time PCR with SYBR Green Supermix (Bio-Rad) and Tc18S-specific oligonucleotides. Data were normalized to murine-GAPDH [17]. Paraffin-embedded tissue-sections (5-micron) were stained with hematoxylin and eosin, and analyzed using an Olympus polarizing microscope system. The presence of inflammatory cells and parasites pseudocysts were scored as described [18]. Masson’s Trichrome-stained tissue-sections (10-sections/mouse) were assessed for fibrosis (blue-colored collagen area) as a percentage of the total myocardial area [12][18].
Statistical analysis
Data are expressed as mean±SD (n=8/group, triplicate observations per experiment). Data were analyzed by the Student t test and 1-way analysis of variance using a SPSS software (v.14.0). Significance is shown by *p<0.05, **p<0.01, or ***p<0.001.
RESULTS
Immune mechanisms elicited by TcG2- and TcG4-encoding DNA/rMVA vaccine
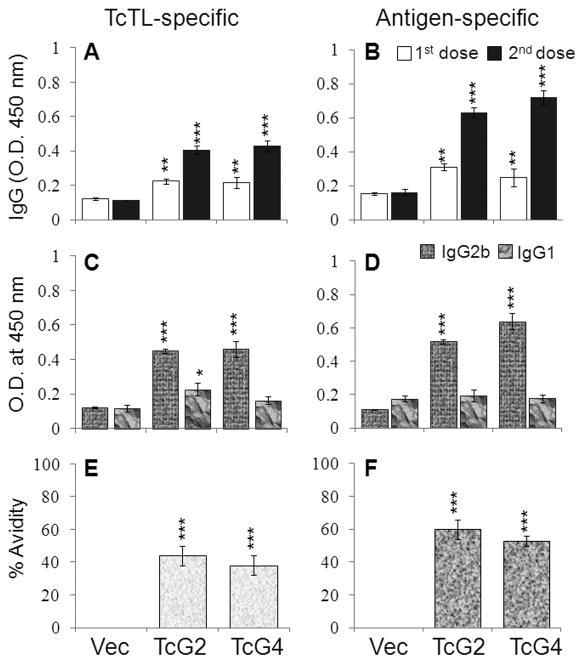
Sera levels of T. cruzi- and antigen-specific IgGs, determined by an ELISA, were detectable after 1st-dose, and enhanced after 2nd-dose (Figs.1A&B, p<0.01–0.001). IgG2a antibodies were not elicited, and IgGs were primarily constituted of IgG2b (IgG2b/IgG1>1, Fig. 1C&D, p<0.001). Further, TcG2- and TcG4-elicited IgGs exhibited a substantial TcTL- and antigen-binding capacity (38–60%, Fig. 1E&F, p<0.001) and trypanolytic activity (86–88% at 1:8 dilution, p<0.05). These data suggested TcG2- and TcG4-encoding DNA/rMVA elicited a strong antigen- and parasite-specific, high avidity, lytic, Th1-type antibody response in mice.
Fig. 1. DNA/MVA delivery of T. cruziantigens induced Th1 pola rized, high-avidity, lytic antibody response in mice.
C57BL/6 mice were immunized with TcG2- and TcG4-encoding DNA/rMVA. Sera levels of T. cruzi (TcTL) and antigen-specific IgGs (A&B) and IgG subtypes (C&D) were determined by an ELISA. (E&F) Percent avidity of vaccine induced IgGs. Data (mean±SD) presented in all figures are representative of three independent experiments (n=4/group/experiment, *p<0.05, **p<0.01, ***p<0.001, immunized-versus-controls).
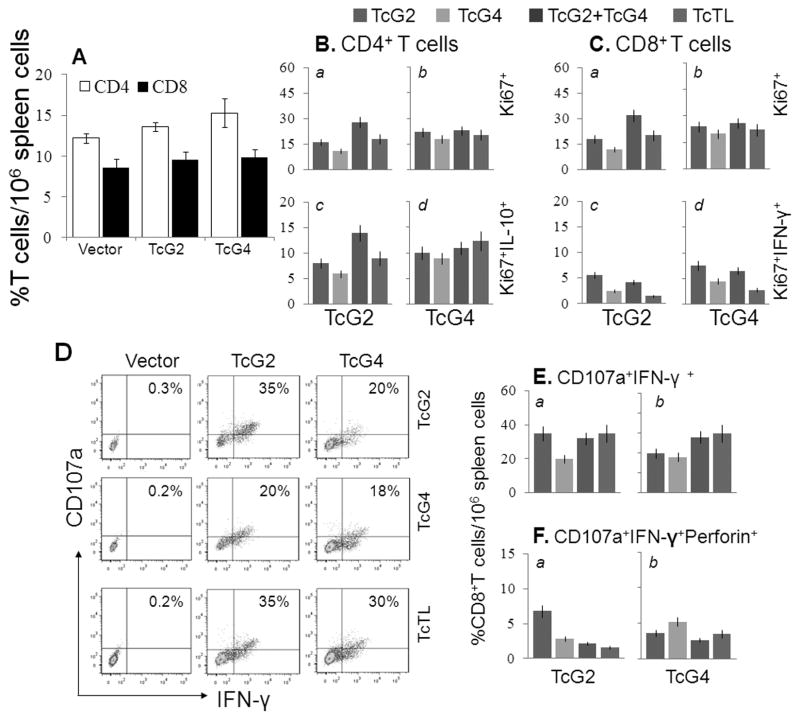
Splenocytes from immunized and control mice were in vitro stimulated with TcTL or recombinant antigens, and cytokine response profiled by an ELISA. Splenocytes of immunized mice exhibited a substantial IFN-γ (112–230-pg/ml) and TNF-α (30–155-pg/ml) release, and low IL-10 release (65–150-pg/ml) (Table S1A, p<0.05–0.001). In vivo percentage of CD4+ (13.6%–15.3%) and CD8+ (9.5%–9.8%) T cells in immunized mice were comparable to controls (Fig. 2A). After in vitro stimulation, CD4+ (Ki67+: 11–28%) and CD8+ (Ki67+: 12–32%) T cells of immunized mice exhibited TcTL- and antigen-specific proliferative capacity, the Ki67+CD4+ cells being mainly IL-10+ (6–14%) and Ki67+CD8+ cells being IFN-γ+ (1.5–7.6%) (Fig. 2B&C). Of the Ki67+CD8+T cells, 18–35% were CD107a+IFN-γ+ (Fig. 2D&E), and a significant proportion exhibited CD107a+perforin+ (1.6–6.8%) phenotype, the higher frequency being observed in TcG2-immunized mice compared to the TcG4-immunized mice (Fig. 2E&F, p<0.05–0.01). The frequency of proliferating and non-proliferating T cells producing other cytokines was very low (<3%) or not observed in immunized mice (data not shown). These results suggested that TcG2- and TcG4-encoding DNA/rMVAs elicited antigen- and parasite-specific CD4/CD8 T cell proliferation. The CD8+T cells were predominantly IFN-γ+ with cytolytic capacity (CD107a+perforin+) and had a potential to act as effector T cells against T. cruzi.
Fig. 2. Splenic characterization of T cell response in immunized mice.
Mice were immunized as in Fig. 1. (A) Splenic T cell profile 2-weeks after 2nd immunization. (B&C) Splenocytes were in vitro stimulated with TcTL or recombinant antigens. Shown are the mean percentage of IL-10+ and IFN-γ+ proliferating (Ki67+) CD4+ and CD8+T cells. (D) Flow cytometry quadrant analysis of CD8+T cells stained for CD107a (Alexa Fluor 488) and IFN-γ (e-Fluor). (E&F) Shown are the percentage of CD8+IFN-γ+ T cells that were CD107a+Perforin− (E) and CD107a+Perforin+ (F).
Expansion of TcG2/TcG4-primed immunity in response to T. cruzi
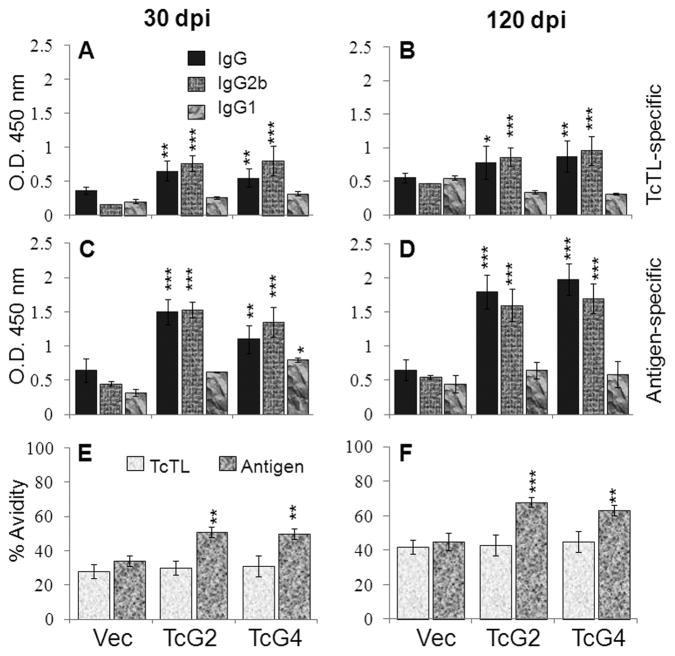
We determined sera (1:1000-dilution) antibody levels at 30-dpi and 120-dpi corresponding to acute and chronic stages of infection and disease development. TcG2- and TcG4-immunized mice exhibited ~10–30-fold expansion of parasite- and antigen-specific IgG (IgG2b>IgG1) response to T. cruzi (compare Fig. 1A–D and Fig. 3A–D). The IgG/IgG2b response in immunized/infected mice was 2–5-fold higher than the infected controls, both at 30- and 120-dpi (Fig. 3A–D, p<0.05–0.001). The antigen-binding capacity of IgGs in immunized/infected mice, though lower than that noted after immunization (compare Fig. 3E&F and Fig. 1E&F), was significantly higher when compared to infected controls (51%-versus-30%, and 63–68%-versus-40%, 30-dpi and 120-dpi, respectively, p<0.05–0.001, Fig. 3E&F). These data suggested that antigen-encoding DNA/rMVAs induced high avidity IgG/IgG2b response expanded in a parasite-specific manner upon challenge infection (TcG2-immunized>TcG4-immunized) and persisted during the chronic phase.
Fig. 3. Expansion of immunization-induced antibodies in response to challenge T. cruzi infection.
Mice were immunized as in Fig. 1, infected with T. cruzi (10,000/mouse), and sacrificed at day 30 and 120 post-infection. The sera levels of T. cruzi- (A&B) and antigen- (C&D) specific IgG and subtypes were measured by an ELISA. (E&F) Avidity index of sera antibodies.
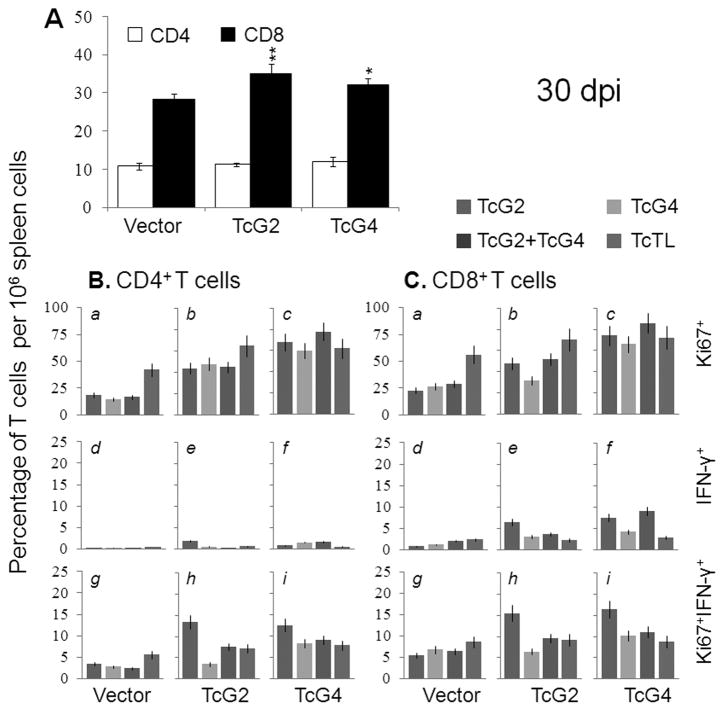
Splenic cells’ functional profile following challenge infection is shown in Fig. 4 and Table S1. Splenocytes of immunized/infected mice exhibited a prolific T. cruzi and antigen-specific activation with substantially higher levels of antigen-specific secretion of cytokines (IFN-γ+TNF-α≫IL-4+IL-10) as compared to those noted in infected controls (Table S1A, p<0.05–0.001). Splenic cytokine response to infection was higher in TcG2-immunized mice as compared to the TcG4-immunized mice (Table S1A). Flow cytometry analysis showed challenge infection resulted in no change in CD4+T cells and ~4-fold and ~3-fold increase in CD8+T cell population in immunized and control mice, respectively (compared Fig. 2A and Fig. 4A). When stimulated in vitro, CD4+ (Ki67+:44–78%) and CD8+ (Ki67+:32–86%) T cells from immunized/infected mice exhibited a prolific TcTL- and antigen-specific proliferation (Fig. 4B&C.a –c). Up to 16%, 12% and 8% of Ki67+CD4+, Ki67+CD8+, and Ki67−CD8+ T cells from immunized/infected mice were IFNγ+ (Fig. 4B&C.d–i, p<0.05). No in situ IL-10 staining was observed for either proliferating or non-proliferating CD8+T cells from immunized/infected mice. In comparison, a moderate-to-low frequency of proliferating and non-proliferating CD4+ and CD8+ T cells with IFN-γ+, TNF-α+ and IL-10+ phenotype were observed in acutely-infected controls (Fig. 4B&C, Table S1B). These results suggested that mice immunized with TcG2- and TcG4-encoding DNA/rMVA responded to challenge infection with a type 1 cytokine response and expansion of IFN-γ+CD8+T cells; the T cell response was more robust in TcG2-immunizedmice.
Fig. 4. Splenic T cell response to challenge infection in immunized mice.
Mice were immunized, infected, and harvested as in Fig. 3. (A) Splenic CD4/CD8 T cell population. (B&C) Splenocytes were in vitro stimulated with TcTL or recombinant antigens were incubated with fluorescent-conjugated antibodies as described in Materials and Methods and analyzed by flow cytometry. Shown are the CD4+ (B) and CD8+ (C) proliferating (Ki67+, panels a–c, g–i), and non-proliferating (Ki67−, panels d–f) T cell subsets and their intracellular cytokine (, IFN-γ: panels d–i)) profile.
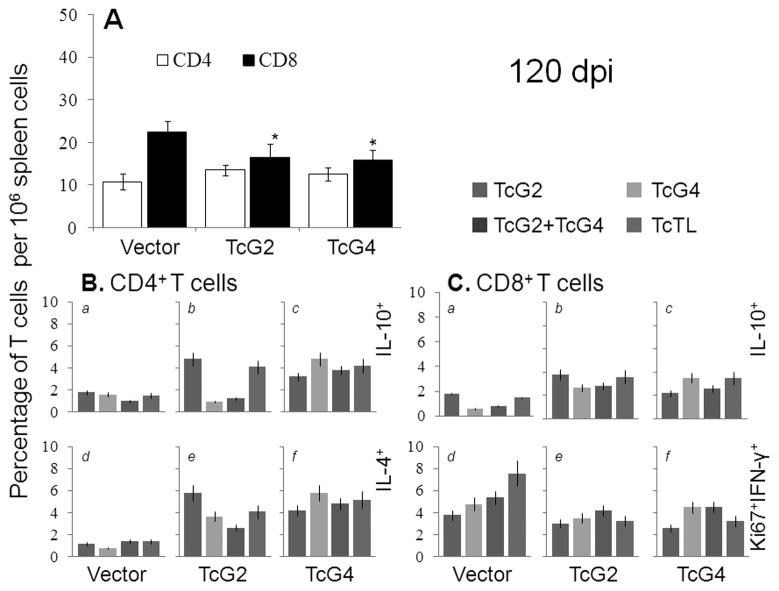
In chronic phase, immunized mice exhibited a massive decline in proinflammatory phenotype. The splenic release of parasite- and antigen-specific IL-4 (85–207-pg/ml) and IL-10 (65–233-pg/ml) was increased and IFN-γ (105–420-pg/ml) and TNF-α (108–362-pg/ml) was decreased in immunized/chronically-infected mice when compared to immunized/acutely-infected mice (Table S1A). The CD4+T cells of immunized/chronically-infected mice were predominantly IL-4+ (~3–6%) and IL-10+ (~2–5%) (Fig. 5B, a–f) with no detection of IFN-γ+ or TNF-α+ phenotype. The CD8+T cells decreased by >50% (Fig. 5A), and subsequently, the frequency of Ki67+IFN-γ+ (~2.5–4%), Ki67+TNF-α+ (1.5–3%) CD8+T cells was significantly decreased, with the emergence of IL-10+CD8+T cells (~3–5%) in chronically-infected/immunized mice as compared to that noted in acutely-infected/immunized mice (compare Fig. 4C&5C, Table S1B). In chronically-infected controls, splenic release of parasite-specific proinflammatory cytokines (IFN-γ: 1002-pg/ml, TNF-α: 505-pg/ml, IL-4: 110-pg/ml, IL-10: 320-pg/ml) and type-1 dominated CD4+/CD8+ T cell profile persisted. These data suggested that mice immunized with TcG2- and TcG4-encoding DNA/rMVA were equipped to control the chronic proinflammatory cytokines and CD8+T cell responses that are considered pathological in Chagas disease.
Fig. 5. Functional profile of splenic T cells in immunized mice chronically infected with T. cruzi.
Mice were immunized and infected as in Fig. 3, and harvested at day 120 post-infection. (A) Splenic frequency of CD4/CD8 T cells. (B&C) Splenocytes were stimulated as in Fig. 4. Shown in (B) are the mean percentage of CD4+T cells that were IL-10+ (a–c) or IL-4+ (d–f); and in (C) the mean percentage of CD8+T cells that were IL-10+(a –c) or Ki67+IFN-γ+ (d–f), measured by flow cytometry.
TcG2- and TcG4-elicited immunity controlled parasite persistence and associated tissue damage
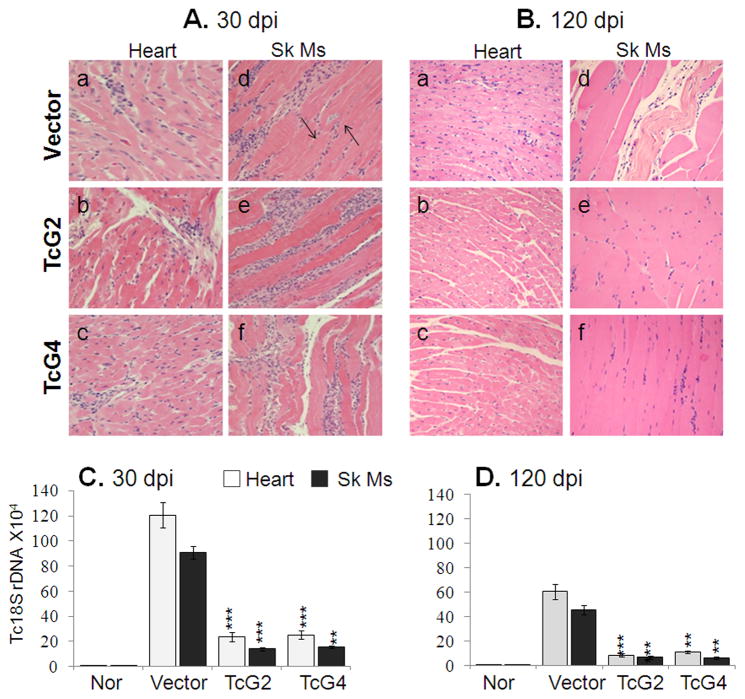
Histological analysis revealed widespread tissue infiltration of inflammatory cells in acutely-infected mice. In agreement with enhanced activation of T cell responses, the tissue infiltration of inflammatory cells in TcG2- and TcG4-immunized/acutely-infected mice was more extensive with coalescing of inflammatory foci or diffused inflammation (score:3–4) than that noted in acutely-infected controls (histological score 1–2, Fig. 6A). Conversely, tissue parasite foci were remarkably reduced (0–3-pseudocysts/microscopic field, mf) in immunized mice while controls presented peak tissue parasite burden (2–6-pseudocysts/mf, Fig. 6A). Real time PCR showed 80–84% reduction in parasite-specific Tc18SrDNA in tissue biopsies of immunized/acutely-infected mice as compared to the controls (Fig. 6C, p<0.05–0.001). In chronic phase, tissue inflammatory infiltrate was dramatically decreased in TcG2- and TcG4-immunized mice (score:0–2) while chronically-infected controls exhibited persistence of diffused inflammatory foci (score:3–4) throughout the heart and skeletal muscle tissue (Fig. 6.B.a–f). Real time PCR showed the parasite-specific Tc18SrDNA signal was decreased by 82–86% in heart and skeletal muscle of immunized mice (Fig. 6D). Chronic tissue fibrosis, extensively observed in heart tissue and skeletal muscle of infected controls by Masson’s-trichrome staining, was decreased by 90–92% in immunized/infected mice (Fig. S1). These results suggested that immunization with TcG2- or TcG4-encoding DNA/rMVA was effective in controlling the tissue parasite burden and, consequently, the associated immunopathology in chronic chagasic mice while in the absence of antigen-elicited immunity, parasite persistence and immunopathology dominated in control mice.
Fig. 6. Control of inflammatory infiltrate, tissue pathology and parasite burden in TcG-2 and TcG4-immunized mice.
(A&B) H&E staining (blue: nuclear, pink: muscle/cytoplasm/keratin) of heart-tissue and skeletal muscle sections from immunized and control mice, harvested at day 30 (A&C) and 120 (B&D) post-infection (magnification: 20X). (C&D) Real time PCR amplification of T. cruzi 18SrDNA sequence, normalized to GAPDH.
DISCUSSION
In the present work, we utilized DNA-prime/MVA-boost approach to deliver the candidate antigens, and investigated the protective efficacy of elicited immune mechanisms against T. cruzi infection and Chagas disease.
Mice immunized with TcG2 and TcG4 elicited a strong parasite and antigen-specific type 1 (IgG2b>IgG1) antibody response that exhibited 38–60% antigen-binding avidity index and 86–88% trypanolytic efficiency, thus, indicating that delivery of antigens by DNA/rMVA approach primed the immunized mice to respond with pathogen-specific effector antibodies. Indeed, challenge infection resulted in a rapid and potent expansion of antibody response in immunized mice. A decline in avidity index of IgGs in immunized/actely-infected mice could be explained by the fact that high avidity IgGs were utilized to remove antigen (T. cruzi), also supported by 82–86% decline in acute parasite burden in immunized mice. The persistence of IgGsin immunizedmice at the onset of chronic phase is similar to the observation of abundant anti-parasite lytic antibodies in clinically-asymptomatic (but not clinically-symptomatic) chagasic patients [19]. Considering the pharmacological half-life of IgGs is ~21-days [20], the long-term presence of high-avidity/lytic IgGs in immunized/chronically-infected mice or human patients indicates ongoing secretion of antibodies from plasma cells or memory B cells differentiation into plasma cells. There is no consensus on whether or not persisting specific antigen is required for B cell maturation and antibody secretion [21,22]. We surmise that TcG2- and TcG4-encoding DNA/rMVAs induced high avidity/lytic antibody response via successfully priming the B cells that was further expanded and was protective against challenge infection. The persistence of antibody response after control of acute infection in immunized mice indicates long-term surveillance by antibodies to ensure parasite control and is beneficial to the host.
Experimental studies suggest that if host is unable to develop a potent type 1 cytokine-secreting CD4/CD8 T cells, and cytotoxic T lymphocytes activity against infection, then a persistence of parasite-induced inflammatory response pursues. A high frequency of inflammatory, especially granzyme+CD8+T cells, is invariably associated with pathology and tissue destruction in chronic chagasic patients [23,24]. Others have shown a correlation between inflammatory cytokine-producing CD4+T cells and clinical disease, and IL-10 production with clinically asymptomatic state in chagasic patients [25,26]. In our study, CD8+T cells primed by immunization with TcG2/TcG4-encoding DNA/rMVAs responded to in vitro and in vivo antigenic stimulation by prolific expansion, and exhibited cytolytic effector phenotype evidenced by increased expression of CD107a, perforin and IFN-γ. Importantly, the proliferation and expansion of effector T cells correlated with >5-fold reduction in acute tissue parasite burden in immunized/infected mice as compared to the controls. A dominant recruitment and proliferation of CD8+T cells (instead of CD4+T cells) upon challenge infection suggests that this subset is primarily involved in the recall response and provided early control of parasite replication in immunized mice. The IFN-γ+CD4+T cells likely assisted the cytotoxic mechanisms of phagocytes and served as helper cells for the development of CD8+ CTL effector cells [27,28].
In the chronic phase (120 dpi), immunized mice exhibited 55–90% reduction in the frequency of type 1 proinflammatory T cells and increased presence of IL-10+ T cells in the lymphoid. Tissue persistence of parasites, inflammatory infiltrate, and fibrosis, the hallmarks of chronic Chagas disease, were decreased by 82–92% in heart and skeletal muscle of TcG2- and TcG4-immunized mice. We surmise that an effective control of acute parasite burden below a threshold level in immunized provided the host ability to prevent T. cruzi-mediated chronic inflammation and tissue damage, and a switch of host immunity to type 2 cytokine and T cell response was indicative of prevention of clinical disease, as is noted in human chagasic patients [29].
Recombinant MVA expressing immunogens from a variety of infectious agents (e.g., HIV, Plasmodium) or tumor-associated antigens have been successfully tested in phase I and II clinical trials [30–33]. In deciding which of our strategies might be more efficacious as a clinical vaccine it is important that the vaccine formulation elicits potent long-term protective immune response, is safe and simple. From our present and previous studies it is clear that candidate antigens, depending upon the method of delivery, elicited different level of protective immunity against T. cruzi (DNA/MVA>DNA/protein>DNA/DNA). The DNA/protein approach provided protective efficacy only when mice were immunized with two doses of TcG1-, TcG2- and TcG4-encoding plasmids + IL-12- and GM-CSF-encoding plasmids followed by two doses of recombinant proteins [12]. We surmise that delivery of TcG2 and TcG4 by DNA/rMVA approach is most efficacious in achieving resistance to T. cruzi infection and chronic disease.
In summary, we have demonstrated that immunization with TcG2- or TcG4-encoding DNA/rMVA enabled the host to control acute T. cruzi infection, and subsequently, prevent the persistent activation and infiltration of inflammatory cells in the heart and skeletal muscle that otherwise occur due to parasite persistence and result in tissue destruction during the chronic phase. Our results provide us impetus to test the efficacy of two-component (TcG2+TcG4) DNA/rMVA vaccine in experimental models and proceed with 1st-phase clinical trial to test the immunogenicity of TcG2/TcG4-encoding DNA/rMVA in humans.
Supplementary Material
DNA-rMVAs-encoding TcG2/TcG4 primed type 1 immunity expanded robustly upon T. cruzi infection.
Immunization with TcG2/TcG4 provided resistance to T. cruzi and chronic myocarditis.
TcG2 and TcG4 are excellent candidates for vaccine against Chagas disease.
Acknowledgments
We are thankful to Dr. Bernard Moss (Laboratory of Viral Diseases at National Institutes of Health, Bethesda, MD) for generously providing the pLW44 vector used in this study.
This work was supported in part by grants from the National Institute of Allergy and Infectious Disease/National Institutes of Health (AI072538, AI054578) and from American Heart Association (0855059F) to NJG. SG is a recipient of Sealy Center for Vaccine Development post-doctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young C, Losikoff P, Chawla A, Glasser L, Forman E. Transfusion-acquired Trypanosoma cruzi infection. Transfusion. 2007;47(3):540–4. doi: 10.1111/j.1537-2995.2006.01147.x. [DOI] [PubMed] [Google Scholar]
- 2.Munoz J, Portus M, Corachan M, Fumado V, Gascon J. Congenital Trypanosoma cruzi infection in a non-endemic area. Trans R Soc Trop Med Hyg. 2007;101(11):1161–2. doi: 10.1016/j.trstmh.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115(9):1109–23. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 4.Bonney KM, Engman DM. Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med. 2008;8(6):510–8. doi: 10.2174/156652408785748004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez-Chagoyan JC, Gupta S, Garg NJ. Vaccine development against Trypanosoma cruzi and Chagas disease. Adv Parasitol. 2011;75:121–46. doi: 10.1016/B978-0-12-385863-4.00006-X. [DOI] [PubMed] [Google Scholar]
- 6.Cazorla SI, Becker PD, Frank FM, Ebensen T, Sartori MJ, Corral RS, et al. Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect Immun. 2008;76(1):324–33. doi: 10.1128/IAI.01163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyahira Y, Takashima Y, Kobayashi S, Matsumoto Y, Takeuchi T, Ohyanagi-Hara M, et al. Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect Immun. 2005;73(11):7356–65. doi: 10.1128/IAI.73.11.7356-7365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia V, Garg NJ. Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin Vaccine Immunol. 2008;15(8):1158–64. doi: 10.1128/CVI.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aparicio-Burgos JE, Ochoa-Garcia L, Zepeda-Escobar JA, Gupta S, Dhiman M, Martinez JS, et al. Testing the efficacy of a multi-component DNA-prime/DNA-boost vaccine against Trypanosoma cruzi infection in dogs. PLoS Negl Trop Dis. 5(5):e1050. doi: 10.1371/journal.pntd.0001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drexler I, Staib C, Sutter G. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr Opin Biotechnol. 2004;15(6):506–12. doi: 10.1016/j.copbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia V, Sinha M, Luxon B, Garg N. Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: In silico and in vitro screening. Infect Immun. 2004;72:6245–54. doi: 10.1128/IAI.72.11.6245-6254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Garg NJ. Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Negl Trop Dis. 4(8):e797. doi: 10.1371/journal.pntd.0000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staib C, Drexler I, Sutter G. Construction and isolation of recombinant MVA. Methods Mol Biol. 2004;269:77–100. doi: 10.1385/1-59259-789-0:077. [DOI] [PubMed] [Google Scholar]
- 14.Boyle JS, Silva A, Brady JL, Lew AM. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc Natl Acad Sci U S A. 1997;94(26):14626–31. doi: 10.1073/pnas.94.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Wen JJ, Dhiman M, Whorton EB, Garg NJ. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 2008;10(10–11):1201–9. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garg N, Bhatia V, Gerstner A, deFord J, Papaconstantinou J. Gene expression analysis in mitochondria from chagasic mice: Alterations in specific metabolic pathways. Biochemical J. 2004;381:743–52. doi: 10.1042/BJ20040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhiman M, Garg NJ. NADPH oxidase inhibition ameliorates Trypanosoma cruzi-induced myocarditis during Chagas disease. J Pathol. 225(4):583–96. doi: 10.1002/path.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordeiro FD, Martins-Filho OA, Da Costa Rocha MO, Adad SJ, Correa-Oliveira R, Romanha AJ. Anti-Trypanosoma cruzi immunoglobulin G1 can be a useful tool for diagnosis and prognosis of human Chagas’ disease. Clin Diagn Lab Immunol. 2001;8(1):112–8. doi: 10.1128/CDLI.8.1.112-118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins RJ, Kramer WG, Blackwelder WC, Ashtekar M, Hague L, Winker-La Roche SD, et al. Safety and pharmacokinetic evaluation of intravenous vaccinia immune globulin in healthy volunteers. Clin Infect Dis. 2004;39(6):759–66. doi: 10.1086/422998. [DOI] [PubMed] [Google Scholar]
- 21.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298(5601):2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 22.Montes CL, Acosta-Rodriguez EV, Merino MC, Bermejo DA, Gruppi A. Polyclonal B cell activation in infections: infectious agents’ devilry or defense mechanism of the host? J Leukoc Biol. 2007;82(5):1027–32. doi: 10.1189/jlb.0407214. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi Mde L, Benvenuti LA, Martins Reis M, Metzger M. Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovasc Res. 2003;60(1):96–107. doi: 10.1016/s0008-6363(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 24.Dutra WO, Menezes CA, Villani FN, da Costa GC, da Silveira AB, Reis D, et al. Cellular and genetic mechanisms involved in the generation of protective and pathogenic immune responses in human Chagas disease. Mem Inst Oswaldo Cruz. 2009;104 (Suppl 1):208–18. doi: 10.1590/s0074-02762009000900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza PE, Rocha MO, Rocha-Vieira E, Menezes CA, Chaves AC, Gollob KJ, et al. Monocytes from patients with indeterminate and cardiac forms of Chagas’ disease display distinct phenotypic and functional characteristics associated with morbidity. Infect Immun. 2004;72(9):5283–91. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza PE, Rocha MO, Menezes CA, Coelho JS, Chaves AC, Gollob KJ, et al. Trypanosoma cruzi infection induces differential modulation of costimulatory molecules and cytokines by monocytes and T cells from patients with indeterminate and cardiac Chagas’ disease. Infect Immun. 2007;75(4):1886–94. doi: 10.1128/IAI.01931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe R, Meagher S, Mullins B. Gamma interferon suppresses acute and chronic Trypanosoma cruzi infection in cyclosporin-treated mice. Infect Immun. 1991;59(5):1633–8. doi: 10.1128/iai.59.5.1633-1638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed SG. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J Immunol. 1988;140(12):4342–7. [PubMed] [Google Scholar]
- 29.Costa GC, da Costa Rocha MO, Moreira PR, Menezes CA, Silva MR, Gollob KJ, et al. Functional IL-10 gene polymorphism is associated with Chagas disease cardiomyopathy. J Infect Dis. 2009;199(3):451–4. doi: 10.1086/596061. [DOI] [PubMed] [Google Scholar]
- 30.Webster DP, Dunachie S, McConkey S, Poulton I, Moore AC, Walther M, et al. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine. 2006;24(15):3026–34. doi: 10.1016/j.vaccine.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 31.Bejon P, Mwacharo J, Kai OK, Todryk S, Keating S, Lang T, et al. Immunogenicity of the candidate malaria vaccines FP9 and modified vaccinia virus Ankara encoding the pre-erythrocytic antigen ME-TRAP in 1–6 year old children in a malaria endemic area. Vaccine. 2006;24(22):4709–15. doi: 10.1016/j.vaccine.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Dorrell L, Williams P, Suttill A, Brown D, Roberts J, Conlon C, et al. Safety and tolerability of recombinant modified vaccinia virus Ankara expressing an HIV-1 gag/multiepitope immunogen (MVA. HIVA) in HIV-1-infected persons receiving combination antiretroviral therapy. Vaccine. 2007;25(17):3277–83. doi: 10.1016/j.vaccine.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Burgers WA, Shephard E, Monroe JE, Greenhalgh T, Binder A, Hurter E, et al. Construction, characterization, and immunogenicity of a multigene modified vaccinia Ankara (MVA) vaccine based on HIV type 1 subtype C. AIDS Res Hum Retroviruses. 2008;24(2):195–206. doi: 10.1089/aid.2007.0205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.