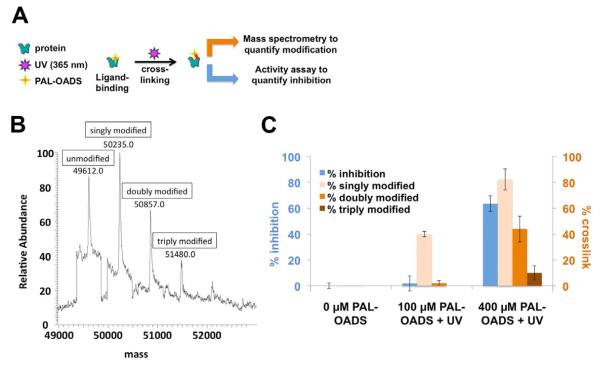
Figure 4. Binding two molecules of OADS per ClC-ec1 protein subunit is necessary and sufficient to inhibit ClC-ec1 function.
A) Experimental schematic: cross-linked protein was subjected to mass spectrometry analysis (as in panel B) and functional studies (reconstitution and Cl−-efflux assays, as in Figure 1 B, C).
B) Covalent modification of ClC-ec1 with 400 μM PAL-OADS detected by mass spectrometry.
C) Comparison of % inhibition of ClC-ec1 activity measured using Cl− efflux (blue bars) to % ClC-ec1 that is singly, doubly and triply modified (light orange, medium orange, and brown bars, respectively). ClC-ec1 in the presence of 0, 100 or 400 μM PAL-OADS was irradiated with 365-nm light UV and then split into two samples as illustrated in panel A. “Singly modified” corresponds to percent ClC-ec1 covalently linked to at least one equivalent of PAL-OADS (sum of singly, doubly, and triply modified protein), detected by mass spectrometry as in panel B. “Doubly modified” = % ClC-ec1 covalently linked to at least two equivalents of PAL-OADS (sum of doubly and triply modified protein). “Triply modified” = % ClC-ec1 covalently linked to three equivalents of OADS. The combined results demonstrate that (1) binding of one OADS molecule to ClC-ec1 does not inhibit ClC-ec1 (middle, results with 100 uM PAL-OADS), (2) binding of two OADS molecules correlates well with inhibition (right, results with 400 μM PAL-OADS), and (3) binding of three OADS molecules is not required for inhibition (right, results with 400 μM OADS). Results represent the mean of three experiments (± SEM).