Abstract
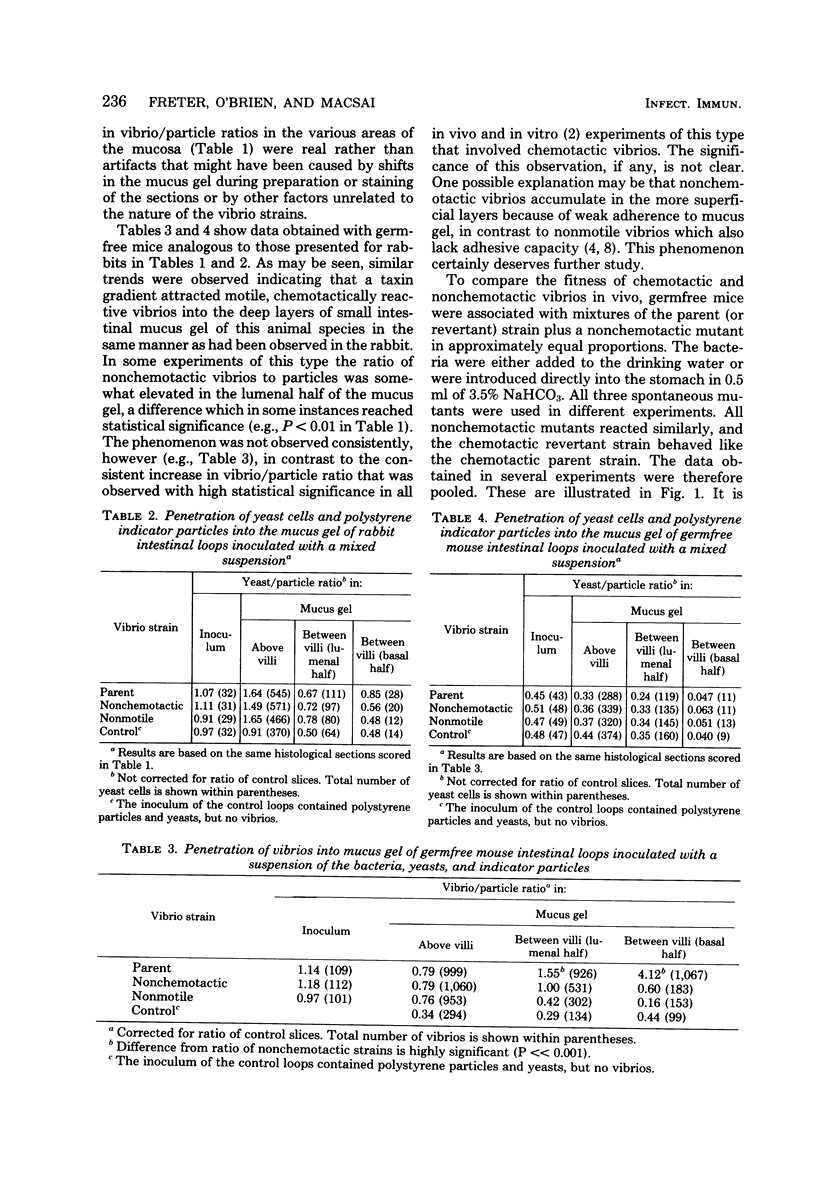
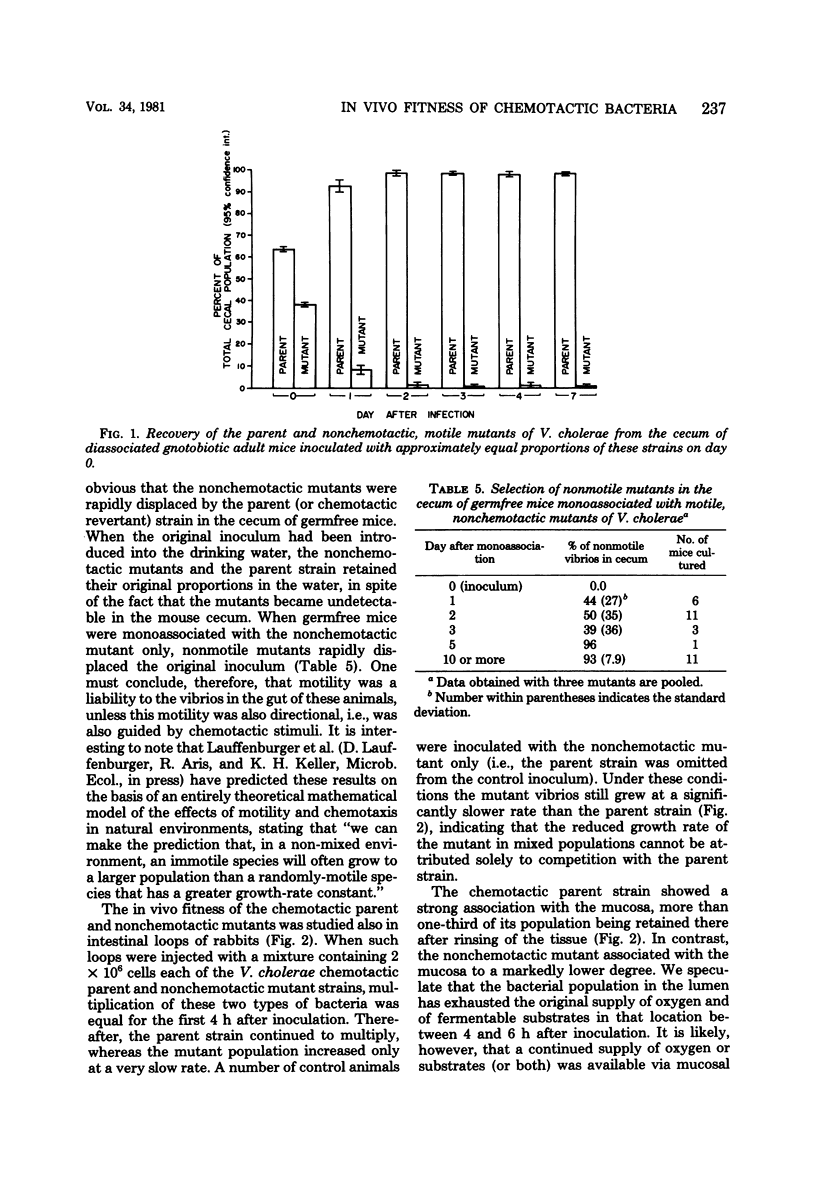
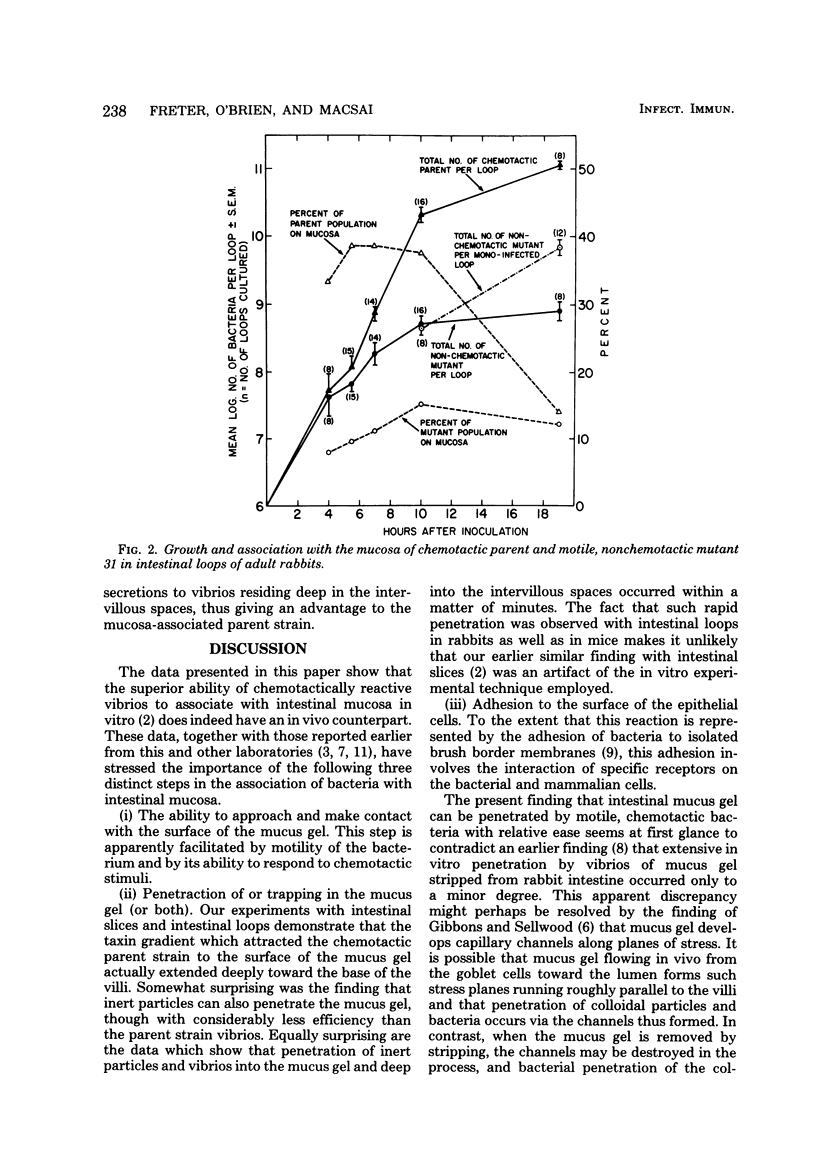
In vivo loops were prepared in the small intestine of rabbits and injected with mixtures of Vibrio cholerae and polystyrene spheres (1.1-micrometers diameter). The loops were removed and frozen after 15 min and then sectioned in a cryostat. The locations of particles and vibrios were determined microscopically. The vibrio/particle ratio was unity in the lumen of the loops, but increased 10-fold in the deep intervillous spaces, indicating active invasion of the mucus gel by the chemotactic parent strain. Motile nonchemotactic mutants and nonmotile mutants of this strain invaded the mucus at the same rate as inert particles. Similar results were obtained with intestinal loops prepared in germfree mice. When germfree mice were disassociated with mixtures of chemotactic (parent or revertant) and nonchemotactic mutant vibrios in equal proportions, the chemotactic strain rapidly outgrew its nonchemotactic counterpart in the intestine. Nonchemotactic mutants introduced as monoassociates into germfree mice were rapidly overgrown by nonmotile mutants which apparently arose spontaneously in the gut. Motility was therefore beneficial to survival only when it was directed by chemotactic stimuli, whereas it was a liability in the absence of such stimuli. Growth of chemotactic vibrios in small intestinal loops of rabbits paralleled that of nonchemotactic mutants for the first 4 to 6 h. Thereafter, the growth rate of the chemotactic vibrios was significantly faster. This was correlated with a significantly higher degree of association with the mucosa on the part of the chemotactic vibrios.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FRETER R. COMPARISON OF IMMUNE MECHANISMS IN VARIOUS EXPERIMENTAL MODELS OF CHOLERA. Bull World Health Organ. 1964;31:825–834. [PMC free article] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun. 1981 Oct;34(1):215–221. doi: 10.1128/iai.34.1.215-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect Immun. 1981 Oct;34(1):222–233. doi: 10.1128/iai.34.1.222-233.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAWA A., FRETER R. ECOLOGICAL MECHANISM CONTROLLING GROWTH OF ESCHERICHIA COLI IN CONTINUOUS FLOW CULTURES AND IN THE MOUSE INTESTINE. J Infect Dis. 1964 Jun;114:235–242. doi: 10.1093/infdis/114.3.235. [DOI] [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkheimer G., Schulz F. H. The phenomenon of persorption. Digestion. 1968;1(4):213–218. doi: 10.1159/000196856. [DOI] [PubMed] [Google Scholar]


