Abstract
Renal cell carcinoma (RCC) is a biologically heterogeneous disease, with many small renal masses (SRMs) exhibiting an indolent natural history, while others progress more rapidly to become life-threatening. Existing multiphase contrast-enhanced imaging methods, such as computed tomography or magnetic resonance imaging, cannot definitively distinguish between benign and malignant solid tumors or identify histologic subtype, and early results of molecular imaging studies (positron emission tomography [PET]) in the evaluation of SRMs have not improved on these established modalities. Alternative molecular markers/agents recognizing aberrant cellular pathways of cellular oxidative metabolism, DNA synthesis, and tumor hypoxia tracers are currently under development and investigation for RCC assessment, but to date none are yet clinically applicable or available. In contrast, immuno-PET offers highly selective binding to cancer-specific antigens, and might identify radiographically recognizable and distinct molecular targets. A phase I proof-of-concept study first demonstrated the ability of immuno-PET to discriminate between clear-cell RCC (ccRCC) and non-ccRCC, utilizing a chimeric monoclonal antibody to carbonic anhydrase IX (cG250, girentuximab) labeled with 124I (124I-girentuximab PET); the study examined patients with renal masses who subsequently underwent standard surgical resection. A follow-up phase III multicenter trial confirmed that 124I-cG250-PET can accurately and noninvasively identify ccRCC with high sensitivity (86%), specificity (87%), and positive predictive value (95%). In the challenge to appropriately match treatment of an incidentally identified SRM to its biological potential, this highly accurate and histologically specific molecular imaging modality demonstrates the ability of imaging to provide clinically important preoperative diagnostic information, which can result in optimal and personalized therapy.
Keywords: kidney, neoplasms, clear cell, diagnosis, molecular imaging, PET, immuno-PET
Introduction
The increased routine use of cross-sectional body imaging has resulted in a dramatic rise in the detection of renal tumors over the last 20 years, and in 2012 an estimated 64,000 men and women will be diagnosed with cancer of the kidney or renal pelvis, most commonly renal cell carcinoma (RCC).1 This increased rate of RCC detection has resulted in a stage migration towards localized tumors less than 7 cm in size (stage I),2,3 most notably in elderly patients.4 Furthermore, the malignant potential of small renal masses (SRMs) is heterogenous, ranging from those that are pathologically benign (20%)5 to those that are anticipated to be aggressive RCC (20%–30%),6,7 and more than 20% of kidney cancer patients ultimately will die from their disease.1 However, while the number of treated SRMs has increased to match this increased incidence and rate of kidney tumor detection, overall RCC mortality rates have remained stable. This finding suggests that early detection of RCC has minimally impacted the absolute number of aggressive lesions that have the potential to cause cancer-related death,8 and has raised concerns that we may be overdiagnosing and overtreating indolent disease.9
Surgical excision via radical nephrectomy (RN) or partial nephrectomy (PN) remains the gold-standard therapy for the stage I renal mass,10,11 and a recent analysis of the Surveillance, Epidemiology, and End Results Program registry data suggested that greater than 95% of patients with SRMs undergo some form of intervention.12 While substantial effort has been directed towards increasing the utilization of nephron-sparing surgical techniques,13,14 the potential of overdiagnosis and overtreatment of clinically insignificant RCC remains of significant concern, particularly in the elderly, in whom the risks of intervention may outweigh any predicted survival benefit.15 As a result, investigators have focused attention on defining the natural history of renal tumors managed expectantly,16,17 and close observation with serial reassessment, ie, active surveillance (AS), has become an accepted management strategy. Recently, AS has become incorporated into the current best-practice guideline for select elderly or comorbid patients who are not fit for immediate surgery.10
The ability to match the level of treatment intensity to tumor biology correctly remains elusive. Multiphase contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) currently provides the best pre-operative assessment of a renal tumor, providing anatomic detail, information on bilateral baseline renal function, and clinical staging. While cross-sectional imaging accurately differentiates between solid and cystic lesions, it provides minimal biological information, and poorly predicts for a tumor’s malignant potential or clinical behavior. These existing imaging methods cannot definitively distinguish between benign and malignant solid lesions, the various RCC histologic phenotypes, or indolent versus aggressive tumors. Molecular imaging has the potential to characterize biological processes at the cellular and subcellular level in a noninvasive fashion, as well as provide the macroscopic detail that is obtained from conventional cross-sectional imaging techniques. Compared to 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography (18F-FDG-PET), which has been shown to have highly variable sensitivity in RCC diagnosis and staging,18 antibody-based molecular imaging (immuno-PET), has demonstrated exciting potential to improve on the existing imaging standard of RCC assessment.19 The most promising results have been reported utilizing 124I-cG250 (girentuximab), a chimeric monoclonal antibody that recognizes carbonic anhydrase (CA) IX,20 which is overexpressed in 93%–97% of clear-cell RCC (ccRCC) tumors.21–23 In this review, we discuss the limitations of conventional imaging, the promise of 124I-cG250 immuno-PET to accurately distinguish ccRCC from non-ccRCC variants in the pretreatment setting, and the potential impact that improved pretreatment tumor characterization and RCC diagnostics may have on contemporary treatment algorithms for SRMs.
Preoperative nonextirpative assessment of malignant potential
Traditionally, contrast enhancement of a solid renal lesion has been considered indicative of malignant pathology. However, a recent systematic review of the literature demonstrated that approximately 15% of SRMs undergoing surgical resection (range 7%–33%) are pathologically benign.24 As a result, a substantial proportion of patients are subject to unneeded risks of morbidity from routine surgery25,26 and from the possible development of chronic kidney disease.27 Under ideal circumstances, the preoperative tumor clinical assessment would adequately stratify patients into risk categories: patients with potentially dangerous but curable RCC warranting immediate intervention; patients at risk for progression who may benefit from neoadjuvant treatment strategies; and patients with benign tumors or indolent disease best suited for AS. Until this is possible, the predictive utility of nonextirpative management involving repeated radiographic assessments, clinical nomograms, and renal mass biopsy will be the focus of further investigation and refinement.
Considerable effort has been expended to identify additional radiographic characteristics associated with aggressive tumor biology and disease progression following treatment. On conventional CT or MRI, while contrast enhancement of a solid lesion is traditionally considered to indicate a malignant lesion until proven otherwise, recent large series have further demonstrated that increasing tumor size is associated with an increased likelihood of malignancy,28,29 high-grade disease,28–30 clear-cell histology,29,30 and presence of synchronous metastases.31–33 Unfortunately, early efforts to develop quantitative predictive nomograms for malignant pathology and tumor grade using radiographic features (tumor size) and clinical variables such as age, sex, smoking history, and symptomatic presentation have been largely unsuccessful and with limited clinical utility.34,35 However, there is also recent evidence suggesting that more detailed tumor anatomic characteristics, such as tumor location,36,37 may provide insight regarding malignant phenotype. Using the RENAL nephrometry score (NS),38 a standardized, easily reproducible, and validated tumor anatomic classification system, Kutikov et al demonstrated NS can differentiate between high- and low-grade lesions, as well as predict tumor histology. By integrating NS with patient characteristics (age, sex), a clinical tool was developed that can predict for RCC histology and grade, and it has been externally validated as having accuracy rates rivaling those of percutaneous biopsy.39,40
Historically, preoperative determination of an enhancing renal mass pathology has been solely possible by percutaneous biopsy, which has traditionally been used sparingly to confirm a suspected non-RCC diagnosis, such as metastatic disease from another primary malignancy, renal abscess, or renal lymphoma.10 As concerns regarding needle-tract tumor seeding have been largely relegated, the role of tumor biopsy has expanded, and it is now a consideration in most SRM treatment-decision algorithms.41 In a systematic review, Lane et al reported biopsy accuracy rates > 80% in the contemporary era for the prediction of malignant versus benign histology.42 Contemporary series utilizing larger-gauge biopsy needles and improved immunohistological techniques have exceeded these results, estimating biopsy to have accuracy rates of greater than 90%, and to have minimal risk of adverse events.43 However, biopsy has recognized limitations, including increased rates of “ nondiagnostic” biopsies in small tumors,44 as well as the inability to determine tumor grade in >50% of cases.45,46 Although beyond the scope of this review, the association between a number of molecular markers and malignant potential are currently under investigation,47 which may ultimately provide the best means to match treatment to RCC phenotype. Until such markers exist, attention will remain focused on means of improving the quality of information yielded by cross-sectional imaging.
Cross-sectional imaging of the small renal mass
Pretreatment imaging provides important staging and anatomic information to the treating physician. Multiphase contrast-enhanced CT is currently recommended by the American Urological Association,10 European Association of Urology,48 National Comprehensive Cancer Network,49 and American College of Radiology50 as the imaging modality of choice in the evaluation of renal lesions, with contrast enhancement (attenuation increase of at least 15–20 Hounsfield units from the corresponding noncontrast image) being the most important criterion for differentiating benign from malignant renal lesions. Adequately designed CT protocols should include precontrast and postcontrast imaging at multiple time points, including a nephrographic phase. More complex multiplanar reconstructions and 3-D volume-rendered images are not required in the routine evaluation, but can be helpful for surgical planning.50 CT is highly sensitive even for small renal masses less than 2 cm, demonstrating increased detection rates compared to MRI due to higher spatial resolution. However, while diagnostic accuracy approaches 95% with emerging technologies such as dual-energy CT,51 conventional CT imaging remains limited for differentiating between malignant and benign cystic lesions, and hypovascular lesions such as papillary RCC, which can commonly demonstrate minimal contrast enhancement.
For indeterminate CT results, pregnancy, or contraindication to the administration of intravenous contrast, MRI may be utilized, and can provide additional information regarding anatomic characteristics, local disease extension, and vena cava thrombus involvement. Although the traditional role of MRI has been to better define equivocal CT findings, evolving functional and advanced imaging techniques, including diffusion-weighted and perfusion-weighted imaging, are expanding the role of MRI in the primary evaluation of renal masses.52 MRI can be superior to conventional CT at detecting perirenal fat or venous invasion, and there is emerging evidence to suggest that discrimination between malignant and benign disease and histologic subtypes may be possible with MRI based on degree of enhancement. In 109 renal lesions (64 patients), Taouli et al compared the diagnostic performance of contrast-enhanced MRI with diffusion-weighted MRI, concluding that apparent diffusion coefficient could be used to differentiate between malignant and benign tumors.53 In a single-institution retrospective series of 112 patients undergoing dynamic contrast-enhanced MRI for renal tumors, Sun et al reported that signal-intensity changes on corticomedullary phase images were an effective parameter for preoperatively distinguishing papillary RCC from ccRCC.54 While intriguing, these findings are preliminary, and further study is required to prospectively validate the role of these emerging MRI techniques in the diagnostic evaluation of SRMs.
Molecular imaging of renal tumors
In addition to providing macroscopic detail, molecular imaging has the potential to characterize biological processes at the cellular and subcellular level in a noninvasive fashion. The use of combined anatomic and functional imaging, such as with PET-CT, has received significant attention in the evaluation of presumed malignancy, particularly with RCC. Although PET-CT does not currently have an established role in the staging of RCC,50 functional imaging provides information regarding tumor metabolism, and it has the potential to increase diagnostic accuracy and impact clinical decision-making for newly diagnosed renal lesions. Further, in the targeted therapy era, molecular imaging shows promise for predicting treatment response, as a greater understanding of underlying biological derangements can be expected to lead to recognition of markers that will be discernable on nuclear cross sectional or functional imaging.55
18F-FDG-PET
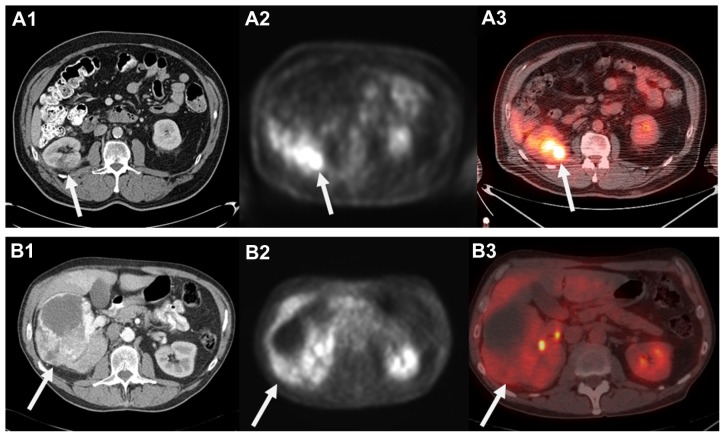
The use of 18F-FDG to functionally image malignancies is based on the presumably altered glycolytic pathway in malignant cells. A glucose analog, 18F-FDG undergoes phosphorylation in metabolically active tumor cells to form 18F-FDG-6-phosphate, which cannot undergo glycolysis and becomes trapped in the cell in higher concentrations than normal tissue. The resulting elevated concentration of 18F-FDG in cells with abnormally high metabolism produces a higher signal relative to the background on PET. The images can be evaluated visually in a qualitative fashion, and semiquantitatively measured by standard uptake value.56,57 While there was initial enthusiasm for the utilization of 18F-FDG-PET to image RCC, there are significant limitations to its clinical utility.18 In the case of most SRMs, the degree of 18F-FDG uptake and limited spatial resolution of contemporary clinical scanners makes it difficult to differentiate tumor from background uptake present in the normal kidney. Further, the normal excretion and accumulation of 18F-FDG in the urinary collecting system and variable levels of uptake that can occur in benign inflammatory and infectious conditions limit its diagnostic accuracy for larger renal lesions (Figure 1).55
Figure 1.
Computed tomography (CT) appearance of small right posterior renal tumor (A1) and corresponding 18F-fluoro-d-glucose positron emission tomography (18F-FDG-PET) (A2) and fused PET low-dose noncontrast CT (A3) images demonstrating FDG uptake. Please note mild right renal uptake related to tracer excretion in the collecting system. In contrast, a 12.5 cm anterior right renal mass clearly visualized on contrast-enhanced CT (B1) that demonstrated minimal to no FDG uptake and was falsely considered benign on PET and fused images (B2 and B3).
Note: These images highlight the lack of sensitivity that has limited the clinical applicability of 18F-FDG-PET in the management of localized renal cell carcinoma.
Lawrentschuk et al recently summarized the collective experience using 18F-FDG-PET as a primary imaging modality to diagnose, stage, or restage RCC. These series are predominantly comprised of a number of small retrospective institutional reports (n = 4–66) performed prior to the advent of combination PET-CT scanning. With 18F-FDG-PET alone, the sensitivity for RCC diagnosis and staging ranged from 32% to 100% and 47% to 75%, respectively, with false-negative rates as high as 68% reported.18 In contrast, while sensitivity is still variable and its clinical application for localized RCC remains unproven, the ability of PET to diagnose regional RCC recurrence or bony, nodal, and visceral metastatic RCC appears to be more specific than with CT alone.58 As a result, there was initial enthusiasm for monitoring disease response to systemic targeted therapy with 18F-FDG-PET.59 However, despite improved survival outcomes with targeted therapy, treatment often results in disease stabilization and tumor necrosis rather than actual tumor regression, which makes response assessment by axial imaging criteria challenging. 60,61 While molecular imaging with 18F-FDG-PET is still being examined as a determinant of treatment response,62–64 low sensitivity rates even in the setting of advanced disease indicate that it is unlikely that 18F-FDG-PET will influence the selection of candidates with metastatic RCC for up-front cytoreductive nephrectomy or consolidative surgical therapy outside of ongoing clinical trials.65
Emerging molecular agents
To overcome the limitations of 18F-FDG-PET, alternative molecular agents associated with aberrant cellular pathways, such as cellular oxidative metabolism, DNA synthesis, and tumor hypoxia tracers, are currently under development and investigation in RCC. Focus has intensified on the use of radiolabeled amino acids, such as 11C-acetate and fluorine- 18 fluorodeoxyglucose, to exploit their increased role in malignant cell metabolism, survival, and proliferation, but clinical utility of these agents are similarly limited by showing low sensitivity with localized disease and short tracer half-lives.66,67 The DNA analog 18F-fluorothymidine, which is phosphorylated by thymidine kinase in the salvage pathway of DNA synthesis, and the nitromidazole 18F-fluoromisonidazole, whose metabolism and tissue retention are dependent on tissue oxygenation, are also currently in the early phases of investigation in PET imaging in patients with RCC; these also may be more promising in the evaluation of metastatic RCC and in measuring response to systemic therapy.68,69
Antibody-based molecular imaging of renal tumors
The discovery of specific tumor targets involved in proliferation, differentiation, angiogenesis, immune recognition, and metastasis over the past decade has facilitated the development of targeted therapeutic agents including monoclonal antibodies and tyrosine kinase inhibitors (TKIs). PET imaging applying radiolabeled monoclonal antibodies and TKIs has potential to better define the biology and efficacy of targeted agents in individual patients, improve the efficiency of drug development, and identify the patients having the best chance to benefit from specific therapy. The ability of PET to quantitatively image the distribution of the radiolabeled agent makes this technique a valuable tool to assess target expression, and accumulation in both tumor lesions and normal tissues in the pretreatment setting followed by imaging during therapy affords the opportunity to demonstrate that tumor targeting is successful.70 In addition to monitoring of response to therapy, molecular imaging also demonstrates potential to characterize in vivo biology for diagnostic purposes, with potential to characterize tumor aggressiveness and optimally guide treatment planning. 55 Currently, a growing number of monoclonal antibodies (U36 [anti-CD44vG], DN30 [anti-cMet], G250 [anti-CA IX], L19-SIP [anti-fibronectin], R1507 [anti-type 1 insulin-like growth factor receptor], J591 and 7E11 [anti-prostate-specific membrane antigen], cetuximab [anti-epidermal growth factor receptor], inbritumumab tiuxetan [anti-CD20], rituximab [anti-CD20], bevacizumab [anti-vascular endothelial growth factor], and trastuzumab [anti-HER2]) have been applied as labeled PET tracers and are currently under investigation in a wide variety of malignancies.70 While a detailed description is beyond the scope of this review, the radiolabeling of small molecules is more challenging and requires a more agent-specific labeling strategy. Although current investigations have been limited to preclinical animal studies, interest in this approach remains high, and a number of protocols have been described that can radiolabel US Food and Drug Administration (FDA)-approved anticancer TKIs.70
To best enable visualization with a PET camera, the agent should be labeled with an inert positron emitter to avoid altering binding properties or pharmacokinetic characteristics. Further, the half-life of the positron emitter should match the agent’s body-residence time as closely as possible. This can range from a few hours for small-molecule agents such as TKIs to several days for monoclonal antibodies.70 The choice of positron emitter is critical to optimize the visualization and quantification of the target of interest, as unbound tracer and other radiolabeled species generated in vivo will increase background activity, reduce the target-background contrast, and decrease overall imaging sensitivity.71 The early development of PET imaging has relied on the use of short-lived nuclides, including 15O, 13N, 11C, and 18F, all with half-lives of a few hours or less. However, recent immuno-PET investigations have utilized antibodies linked to less specific positron-emitting tracers such as iodine-124 (124I) or zirconium-89 (89Zr), which have longer half-lives (days as opposed to hours) that more closely match the pharmacokinetics of intact antibodies than conventional PET radiotracers.18 Disadvantages of the use of monoclonal antibodies for confirmation of target expression include a long body-residence time (which can range from days to weeks) and large molecular size, which can limit tracer diffusion and uptake.70 Evaluation of antibody-based PET probes having faster pharmacokinetics, such as monoclonal antibody fragments and bioengineered proteins, is currently under investigation.72
G250 and carbonic anhydrase IX
First reported in 1986, G250 is a murine monoclonal antibody (mAbG250) developed by immunization of nude mice with human RCC homogenates.73 Subsequently, on isolation and sequential analysis, the G250 antigen was shown to be homologous to the carbonic anhydrase isoenzyme 9 MN/CA IX antigen.74 Early studies investigating the effects of direct mAbG250 treatment in mice with RCC xenografts,75 as well as after vaccination with internal image anti-idiotype antibodies against mAbG250,76,77 resulted in significant antitumor effects, demonstrating promise for CA IX as a therapeutic target. Since administration of the mAbG250 resulted in the formation of human antimouse antibodies,78 a chimeric (cAbG250) immunoglobulin G1 was constructed to reduce its immunogenicity and facilitate repeated injections, with minimal adverse events demonstrated in a dose-escalation trial.79
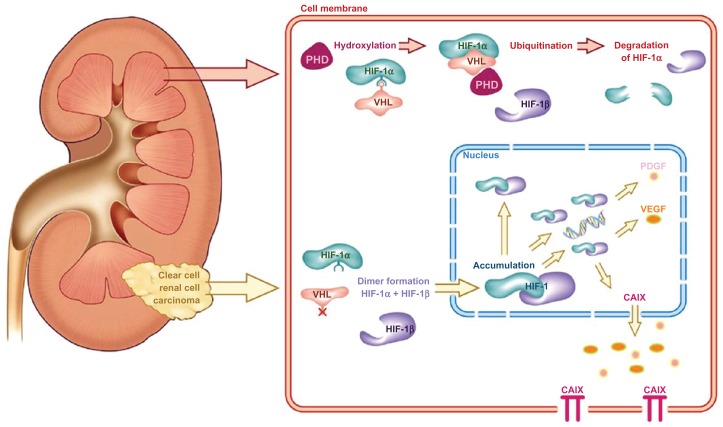
CA IX is a cytosolic and transmembrane enzyme that is active in the adaptation of tumors to hypoxic conditions by regulating the pH of the intracellular and extracellular compartment; CA IX catalyzes the reversible conversion of carbon dioxide and water to carbonic acid. Mediated via hypoxia-inducible factor (HIF)-1α, CA IX overexpression is thought to contribute to an acidic tumor microenvironment to promote cancer progression and metastasis.80,81 Under normal oxygen conditions, HIF-1α is hydroxylated via binding to the Von Hippel–Lindau protein (pVHL), resulting in its ubiquination and controlled degradation. Under hypoxic conditions, pVHL–HIF-1α binding is inhibited, leading to accumulation of HIF-1α and formation of the HIF-1α–HIF-1β complex (Figure 2). This results in the downstream transcription of a number of hypoxia-inducible factors that promote angiogenesis, including CA IX.82 A mutational loss of pVHL, commonly seen in RCC, results in a cellular microenvironment that mimics hypoxic conditions, which explains the routinely high expression of the CA IX antigen in ccRCC.81 Studies confirm that CA IX expression in normal tissue is minimal, limited to the gastrointestinal tract, gallbladder, and pancreatic ducts, while it is expressed in a number of malignant conditions, including gynecologic, gastrointestinal, breast, skin, and genitourinary cancers.83,84
Figure 2.
The Von Hippel–Lindau/hypoxia-inducible factor oxygen-sensing pathway and its relevance in clear-cell renal cell carcinoma.
Note: Copyright © 2010, Elsevier. Reproduced with permission from Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol. 2010;58(1):75–83.81
Abbreviations: PHD, prolyl 4-hydroxylase domain; HIF, hypoxia-inducible factor; VHL, Von Hippel–Lindau; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; CAIX, carbonic anhydrase IX.
CA IX expression has never been reported in noncancerous renal tissue,85 and a review of the literature suggests overexpression of CA IX in >95% of ccRCC tumors.81 Further, CA IX is rarely expressed in non-ccRCC variants, including papillary, chromophobe, and oncocytic tumors.21 The prognostic value of CA IX expression in ccRCC has been extensively investigated, and although some conflicting evidence exists,23 increased CA IX expression is associated with worse overall survival21,86,87 and response to interleukin- 2 immunotherapy.22,88 Based on this data, CA IX tumor expression has been incorporated into clinical nomograms to predict disease-free survival for patients with localized and metastatic RCC, albeit with mixed results.89,90 Although further work remains to better define the relationship between pVHL, HIF-1α, and CA IX expression,80 the highly specific overexpression of CA IX in ccRCC makes it an excellent candidate for further evaluation as a prognostic biomarker, therapeutic target, and molecular imaging agent.
RCC-directed imaging with labeled G250
Due to its high affinity for the CA IX antigen (Ka = 4 × 109 M−1), which is not expressed in normal kidney, the utility of G250 for RCC-directed imaging has been investigated in a number of tumor-targeting studies (Table 1). In a phase I dose-escalation study of intravenously administered 131I-labeled mAbG250 in 16 presurgical patients with RCC, Oosterwijk et al reported that definitive tumor images were observed in twelve patients with G250-positive tumors and one of three patients with G250-negative tumors using single-photon radioimmunoscintigraphic (RIS) techniques.91 Overall, 90% of primary tumors and metastatic RCC lesions were visualized. A subsequent phase I study investigated a single intravenous administration of 131I-cAbG250 at five escalating dose levels, ranging from 2 to 50 mg, in 16 patients undergoing surgical treatment. This study also reported clear visualization of all primary tumors and documented metastatic lesions,79 and although highly heterogeneous, focal 131I-cAbG250 uptake was as high as 0.52% ID/g in primary tumors, with minimal uptake noted in nontumor tissues.
Table 1.
Renal cell carcinoma-directed imaging using radiolabeled G250
| Study | Year | Trial design | Primary outcomes | Patients n (n final) | Imaging agent | RCC stage | Accuracy (primary/mRCC) | Adverse events† |
|---|---|---|---|---|---|---|---|---|
| Oosterwijk et al91 | 1993 | I* | Imaging and biodistribution characteristics | 16 (15) | 131I-mG250 | All | 12/12 ccRCC 1/3 non-ccRCC >90% of primary and mRCC lesions |
None |
| Steffens et al79 | 1997 | I* | Pharmacokinetics, biodistribution, immunogenicity, and imaging characteristics | 16 | 131I-cG250 | All | 13/13 ccRCC 0/3 non-ccRCC 5/5 mRCC |
HACA 2/16 |
| Divgi et al78 | 1998 | I/II | Maximum tolerated dose and therapeutic efficacy (RIT) | 33 | 131I-mG250 | IV | 100% lesions > 2 cm | None |
| Steffens et al99 | 1999 | I | Maximum tolerated dose (RIT) | 12 | 131I-cG250 | IV | Metastases visualized in 9/12 patients | None |
| Brouwers et al92 | 2002 | Open, nonrandomized | Diagnostic efficacy (intrapatient comparison) | 20 | 131I-cG250 vs 18F-FDG | IV | Metastases visualized in 77/112 18F-FDG 34/112 131I-cG250 |
None |
| Brouwers et al95 | 2003 | Subanalysis of phase I/II trial | Diagnostic efficacy (intrapatient comparison) | 5 | 131I-cG250 vs 111In-cG250 | IV |
111In-cG250 47 lesions 131I-cG250 30 lesions |
None |
| Divgi et al100 | 2004 | I | Maximum tolerated dose (fractionated RIT) | 15 | 131I-cG250 | IV | 100% lesions > 2 cm | 2/15 HACA 1/15 G2 allergic reaction |
| Brouwers et al94 | 2005 | I/II | Safety and therapeutic efficacy (RIT) | 29 | 131I-cG250 | IV | CT/MRI 92 lesions 131I-cG250 138 lesions |
None |
| Divgi et al20 | 2007 | I* | Characterization of tumor histology | 26 (25) | 124I-cG250 | All | 15/16 ccRCC 0/9 non-ccRCC Sensitivity 94% NPV 90%, PPV 100% |
None |
| Uzzo et al108 | 2010 | III* | Characterization of tumor histology, comparison of diagnostic efficacy with CECT | 204 (195) | 124I-cG250 | All | 86% sensitivity 87% specificity 95% PPV |
13.3% transient AEs 1/204 G3 hepatic dysfunction 56/198 HACA |
Notes:
Presurgical imaging;
only adverse events related to the use of G250 for diagnostic or pretreatment purposes were reported.
Abbreviations: RCC, renal cell carcinoma; ccRCC, clear-cell RCC; mRCC, metastatic RCC; m, murine; c, chimeric; HACA, human antichimeric antibodies; CECT, contrast-enhanced computed tomography; RIT, radioimmunotherapy; G, grade; NPV, negative predictive value; PPV, positive predictive value; AE, adverse event.
In a phase I/II trial designed to determine the maximum tolerated dose and therapeutic potential of 131I-mAbG250, Divgi et al reported visualization of all lesions ≥ 2 cm in diameter in 33 patients with known metastatic RCC, while lesions < 2 cm were poorly visualized. All patients in this trial developed human mouse antibodies after 4 weeks of therapy, precluding the possibility of repeat treatment.78 To compare the efficacy of 131I-cAbG250 RIS and 18F-FDG-PET in detection of metastatic disease, Brouwers et al compared the two nuclear imaging techniques with conventional cross-sectional imaging in 20 patients with metastatic RCC. Of the identified 112 metastatic lesions, 18F-FDG-PET detected 69%, while 131I-cAbG250-RIS detected only 30%.92 In contrast to prior studies, RIS was the least efficient in visualization of metastatic disease sites, and the authors concluded that 131I-cAbG250-RIS may be more appropriate to identify patients who may benefit from targeted radiotherapy rather than as an assessment of metastatic disease extent. However, in subsequent trials, while not all documented sites showed evidence of 131I-cAbG250 uptake, new metastatic lesions were often found that were unrecognized on CT or MRI, indicating a potential role for patients with suspicious but indeterminate lesions that are apparent on conventional imaging.93–95 It is important to place into context that the objective of measuring tumor targeting with G250 in these studies was not solely for diagnostic purposes, but also as a means to guide the development of G250 targeting for therapeutic purposes. Currently, unlabeled and labeled (using a number of different metallic radionuclides) G250 alone, as well as in unfractionated or fractionated form in combination with interleukin-2, interferon-γ, interferon-α-2a, dendritic cells, and radiotherapy, are currently under investigation for treatment of RCC.78,93,94,96–104 With the exception of an early trial showing evidence of transient liver dysfunction,78 these trials have shown minimal toxicity and low levels of cross-reactivity of G250 with normal tissues, suggesting its potential as a therapeutic agent in the adjuvant setting.81
Diagnostic G250 immuno-PET for the SRM
G250 demonstrates a highly accurate ability to target CA IX-expressing ccRCC. While evidence to suggest a therapeutic role for G250, either alone or as combination therapy, is still accumulating, this ability to discriminate between SRM histologic subtypes in a noninvasive fashion has significant clinical implications and is currently being evaluated in phase III trials. Contemporary PET provides specificity and sensitivity not achieved using other imaging techniques, enabling in vivo molecular imaging of suspected malignancy and comparison with normal tissues. However, the historic limitation of PET and other nuclear imaging techniques as stand-alone imaging applications has been poor anatomic resolution, often making exact localization of a lesion difficult. The combined application of PET and cross-sectional imaging has markedly improved both the localization of lesions and the diagnostic accuracy of either modality in patients with a number of solid tumors (including prostate, pancreas, liver, breast, lung, thyroid, among others), when compared as stand-alone imaging studies.105
Early animal models demonstrated that the cG250 antibody can be stably labeled with a number of positron emitters, such as 89Zr and 124I, without causing loss of antigen affinity. 106 With a 4-day half-life, 124I facilitates the assessment of long-term antibody pharmacokinetics,107 and is currently approved by the FDA for use in clinical trials. In an open- label, phase I proof- of-concept study designed to evaluate the ability to distinguish between ccRCC and non-ccRCC variants, 26 patients with renal masses scheduled for surgical resection (PN or RN with or without lymph-node dissection) were given a single 185 MBq/10 mg (5 mCi/10 mg) intravenous dose of 124I-cG250 in 50 mL of 5% human serum albumin over 20 minutes, 1 week prior to surgery. For each patient, PET and CT images were obtained preoperatively, on the day before or day of surgery. One patient received immunologically inactive (immunoreactivity < 25%) 124I-cG250 and was excluded from analysis. Of the 16 patients with pathologically confirmed ccRCC, 15 had a positive PET (the 16th had necrotic tumor), for a positive predictive value of 100%, sensitivity of 94%, and specificity of 100%.20 Patients with non-ccRCC histologies demonstrated an absence of antibody accumulation in their tumors, and there was no evidence of immediate or delayed toxicity following 124I-cG250 administration.
Based on these encouraging findings, a comprehensive, multicenter, open-label phase III comparative study of PET-CT versus diagnostic CT for the detection of ccRCC in the pretreatment setting was undertaken (Redect; NCT00606632). This trial recently completed enrollment, and the initial results were disseminated at the American Urological Association meeting in 2010.108 A total of 226 patients were enrolled, 204 of which underwent 5 mCi/13.7 mg 124I-girentuximab intravenous infusion, 202 underwent surgical resection, and 195 ultimately met final inclusion criteria for data analysis. PET-CT images were obtained 2–6 days following antibody administration and prior to surgical therapy. Multiphase contrast-enhanced abdominal CTs were performed for comparison within 48 hours of 124I-girentuximab PET-CT. 124I-girentuximab PET-CT was able to discriminate ccRCC from non-ccRCC with much higher sensitivity (86%) and specificity (87%) than conventional CT ( sensitivity 76%, specificity 47%) and had no associated serious adverse events.108 Moreover, 124I-girentuximab PET-CT exhibited a 95% positive predictive value, indicating that a positive test was highly indicative of ccRCC phenotype. These findings, which currently are in press, have led to considerable enthusiasm regarding the potential of a first-in-class histology-specific diagnostic imaging modality (Figure 3). In comparison to previous efforts demonstrating only questionable or theoretical utility with molecular imaging to impact treatment, the Redect trial findings demonstrate that immuno-PET results can provide important pretreatment diagnostic information that will directly impact a patient’s subsequent treatment. Future additional potential 124I- girentuximab PET-CT applications include use in the staging of metastatic disease to measure treatment response, confirm ablation success, and to improve on the appropriate selection of candidates for AS protocols.55
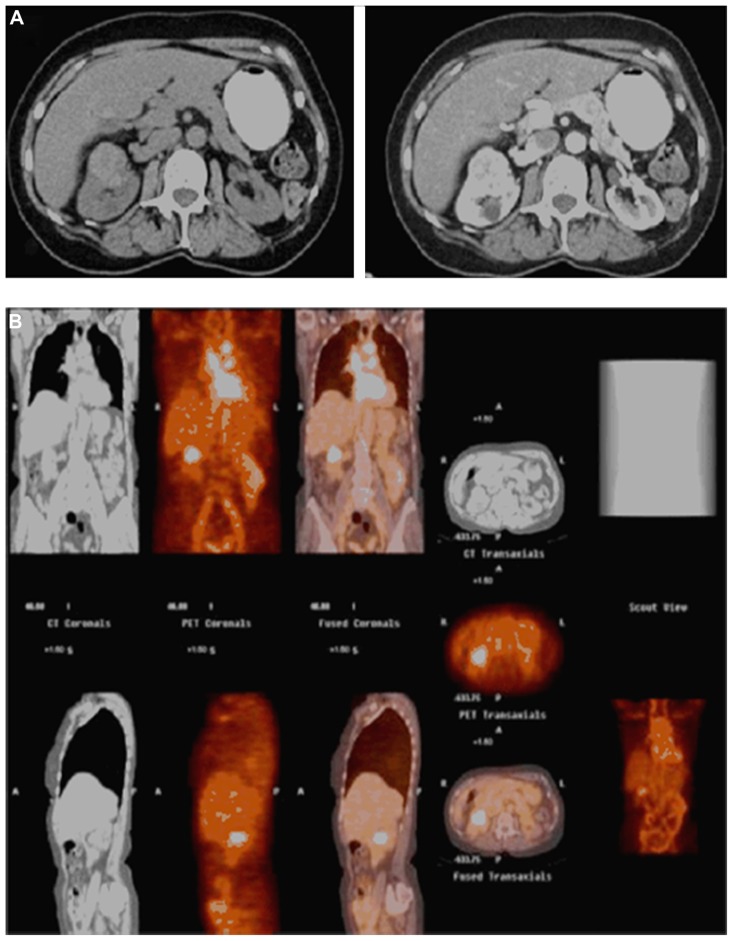
Figure 3.
A 71-year-old female who presented with an incidentally diagnosed, enhancing 4 cm right renal mass (A), which demonstrated positive uptake on 124I-girentuximab (G250) positron emission tomography/computed tomography (B); pathology following open partial nephrectomy revealed pathologic stage T1b Nx Mx clear cell renal cell carcinoma.
Note: Copyright © 2011, Elsevier. Reproduced with permission from Smaldone MC, Chen DY, Uzzo RG, Yu M. Molecular imaging of the small renal mass. Urol Oncol. 2011;29(6):589–592.55
Clinical implications
Localized RCC is a surgically curable disease, with 5-year disease-specific survival rates in excess of 95% for patients having localized tumors treated by RN or PN.109 However, there is increasing evidence to suggest that we are overdiagnosing and overtreating many incidentally detected SRMs, and that a substantial proportion of these likely represent indolent and clinically insignificant tumors. Aggressive surgical resection has remained the gold standard for stage I tumors, in part to ensure treatment of the 20%–30% of small tumors that are estimated to have an aggressive malignant potential. However, reflexive treatment of all SRMs has likely resulted in the overtreatment of many tumors that are not life-threatening, of which at least 15%–20% are pathologically benign.24 This understanding has led to recent interest to characterize the natural history of SRMs that are managed expectantly,16,17 as well as to develop tools that might predict a renal tumor’s malignant potential based on pretreatment patient and imaging characteristics.
To date, currently available molecular imaging has not made a significant impact on the management of localized RCC, largely due to poor sensitivity rates for diagnosing and staging small tumors, compared to existing conventional imaging techniques.18 While there is increased promise for the use of 18F-FDG-PET in the evaluation and monitoring of patients with advanced RCC, it is not recommended in the routine evaluation of the patient presenting with a localized SRM. In contrast, immuno-PET with cG250 offers the ability to discriminate between ccRCC and non-ccRCC phenotypes in the pretreatment setting, with accuracy rates rivaling if not surpassing contemporary percutaneous biopsy series.20,108 Limitations of this emerging imaging technology include greater radiation exposure for the patient, technicians, and family members due to the prolonged half-life of I-124, as well as longer imaging duration compared to more traditional imaging techniques (124I-girentuximab-PET-CT was performed a mean of 5 ± 2 days following tracer injection in the Redect trial).108 Further, concerns regarding how CA IX expression in normal tissues will impact the diagnostic efficacy with 124I-girentuximab PET-CT imaging23 have been largely dismissed. Studies have revealed that in non-ccRCC normal tissues and cell lines, CA IX expression is less homogenous and has lower antigen density per cell when compared to ccRCC tumors.73 This is likely related to functional loss of the VHL gene resulting in CA IX overexpression in ccRCC, in comparison to a locoregional hypoxia mechanism in non-ccRCC tumors and normal tissues.110 The challenge in implementing this novel clinical tool is determining the situation where the result is of maximum benefit. While ccRCC has been shown to have less favorable outcomes compared to papillary and chromophobe variants,111 current guidelines recommend PN for all lesions when possible in appropriate surgical candidates, regardless of tumor histology or grade.10,48 Further, up-front knowledge of histologic type is unlikely to impact treatment decisions in young healthy patients, for whom even a small possibility of metastatic potential would warrant surgical resection, or in elderly or comorbid patients that are not fit to proceed with surgery.
For patients with localized SRMs, immuno-PET is expected to be most useful for patients with poor preoperative renal function, a solitary kidney with a renal mass, bilateral/multifocal disease, or complex cystic disease. Knowledge of histologic type may encourage a nephron-sparing surgical approach compared to radical nephrectomy for complex tumors. Furthermore, the ability to noninvasively determine SRM histology may support a plan of deferring immediate treatment if a non-ccRCC is suggested. While ccRCC is among the most aggressive RCC subtypes and a positive cG250 immuno-PET result might confirm the need for resection, it is important to consider that some immuno-PET-negative patients may still benefit from definitive treatment. Percutaneous biopsy will still play a definitive role in differentiating between benign renal tumors and other more aggressive, non-ccRCC phenotypes, such as papillary type II RCC. For patients presenting with advanced or metastatic RCC, cG250 immuno-PET may play a role in detection of metastases, selecting patients for systemic therapy prior to cytoreductive surgery, guiding selection and optimal duration of systemic therapy, and monitoring treatment response.
Conclusion
Molecular imaging of the SRM provides the potential to characterize biological processes at the cellular level in a noninvasive fashion. Although the poor diagnostic and staging sensitivity of 18F-FDG-PET has dampened enthusiasm for its use in localized RCC, its utility for assessing metastatic tumor burden and efficacy of targeted therapy in advanced disease is currently under investigation. More recently, contemporary studies investigating novel radiopharmacologic and immune- specific agents have demonstrated encouraging potential to improve molecular imaging in RCC. CA IX, which is highly and specifically expressed in ccRCC, has been extensively investigated as both a prognostic marker and therapeutic target. Early clinical experiences demonstrated that cG250 (girentuximab), a monoclonal antibody to CA IX, avidly targets ccRCC, and this affinity has diagnostic and therapeutic applications. Two recent trials have demonstrated that immuno-PET using cG250 ( girentuximab) can accurately and reliably discriminate between ccRCC and non-ccRCC tumor phenotypes with higher sensitivity and specificity than current modalities. In contrast to other molecular imaging techniques, cG250 immuno-PET demonstrates the potential to consistently provide critical information that can directly influence the management of patients presenting with localized renal tumors.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer. 2008;113(1):78–83. doi: 10.1002/cncr.23518. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Mallin K, Ritchey J, Villalta JD, Carroll PR, Kane CJ. Decreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004. J Urol. 2008;179(6):2131–2135. doi: 10.1016/j.juro.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 4.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51(2):203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 5.Kutikov A, Fossett LK, Ramchandani P, et al. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68(4):737–740. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Crispen PL, Boorjian SA, Lohse CM, et al. Outcomes following partial nephrectomy by tumor size. J Urol. 2008;180(5):1912–1917. doi: 10.1016/j.juro.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 7.Remzi M, Ozsoy M, Klingler HC, et al. Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol. 2006;176(3):896–899. doi: 10.1016/j.juro.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98(18):1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 9.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182(4):1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59(4):543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Villalta JD, Meng MV, Whitson JM. Evolving practice patterns for the management of small renal masses in the USA. BJU Int. 2012;110(8):1156–1161. doi: 10.1111/j.1464-410X.2012.10969.x. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67(2):254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smaldone MC, Egleston B, Uzzo R, et al. Does partial nephrectomy result in a durable overall survival benefit in the elderly? J Urol. 2012;187(4):e449. doi: 10.1016/j.juro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma – a meta-analysis and review. J Urol. 2008;179(4):1227–1233. doi: 10.1016/j.juro.2007.11.047. discussion 1233–1234. [DOI] [PubMed] [Google Scholar]
- 17.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118(4):997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrentschuk N, Davis ID, Bolton DM, Scott AM. Functional imaging of renal cell carcinoma. Nat Rev Urol. 2010;7(5):258–266. doi: 10.1038/nrurol.2010.40. [DOI] [PubMed] [Google Scholar]
- 19.Lawrentschuk N, Davis ID, Bolton DM, Scott AM. Positron emission tomography (PET), immuno-PET and radioimmunotherapy in renal cell carcinoma: a developing diagnostic and therapeutic relationship. BJU Int. 2006;97(5):916–922. doi: 10.1111/j.1464-410X.2006.06125.x. [DOI] [PubMed] [Google Scholar]
- 20.Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol. 2007;8(4):304–310. doi: 10.1016/S1470-2045(07)70044-X. [DOI] [PubMed] [Google Scholar]
- 21.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9(2):802–811. [PubMed] [Google Scholar]
- 22.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11(10):3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 23.Leibovich BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25(30):4757–4764. doi: 10.1200/JCO.2007.12.1087. [DOI] [PubMed] [Google Scholar]
- 24.Russo P, Uzzo RG, Lowrance W, et al. Incidence of benign versus malignant renal tumors in selected studies. J Clin Oncol. 2012;30(Suppl 5):357. [Google Scholar]
- 25.Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178(1):41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Simhan J, Smaldone MC, Tsai KJ, et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol. 2011;60(4):724–730. doi: 10.1016/j.eururo.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7(9):735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170(6 Pt 1):2217–2220. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 29.Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol. 2009;181(5):2033–2036. doi: 10.1016/j.juro.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman J, Egleston B, Wong YN, Iffrig K, Lebovitch S, Uzzo RG. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysis. J Urol. 2009;181(1):29–33. doi: 10.1016/j.juro.2008.09.009. discussion 33–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunkle DA, Crispen PL, Li T, et al. Tumor size predicts synchronous metastatic renal cell carcinoma: implications for surveillance of small renal masses. J Urol. 2007;177(5):1692–1696. doi: 10.1016/j.juro.2007.01.029. discussion 1697. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen MM, Gill IS. Effect of renal cancer size on the prevalence of metastasis at diagnosis and mortality. J Urol. 2009;181(3):1020–1027. doi: 10.1016/j.juro.2008.11.023. discussion 1027. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182(1):41–45. doi: 10.1016/j.juro.2009.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeldres C, Sun M, Liberman D, et al. Can renal mass biopsy assessment of tumor grade be safely substituted for by a predictive model? J Urol. 2009;182(6):2585–2589. doi: 10.1016/j.juro.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178(2):429–434. doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 36.Schachter LR, Bach AM, Snyder ME, Kattan MW, Russo P. The impact of tumour location on the histological subtype of renal cortical tumours. BJU Int. 2006;98(1):63–66. doi: 10.1111/j.1464-410X.2006.06179.x. [DOI] [PubMed] [Google Scholar]
- 37.Venkatesh R, Weld K, Ames CD, et al. Laparoscopic partial nephrectomy for renal masses: effect of tumor location. Urology. 2006;67(6):1169–1174. doi: 10.1016/j.urology.2006.01.089. discussion 1174. [DOI] [PubMed] [Google Scholar]
- 38.Kutikov A, Uzzo RG. The RENAL nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Kutikov A, Smaldone MC, Egleston BL, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol. 2011;60(2):241–248. doi: 10.1016/j.eururo.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HK, Zhu Y, Yao XD, et al. External validation of a nomogram using RENAL nephrometry score to predict high grade renal cell carcinoma. J Urol. 2012;187(5):1555–1560. doi: 10.1016/j.juro.2011.12.099. [DOI] [PubMed] [Google Scholar]
- 41.Volpe A, Finelli A, Gill IS, et al. Rationale for percutaneous biopsy and histologic characterisation of renal tumours. Eur Urol. 2012;62(3):491–504. doi: 10.1016/j.eururo.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy – a renaissance? J Urol. 2008;179(1):20–27. doi: 10.1016/j.juro.2007.08.124. [DOI] [PubMed] [Google Scholar]
- 43.Wang R, Wolf JS, Jr, Wood DP, Jr, Higgins EJ, Hafez KS. Accuracy of percutaneous core biopsy in management of small renal masses. Urology. 2009;73(3):586–590. doi: 10.1016/j.urology.2008.08.519. discussion 590–591. [DOI] [PubMed] [Google Scholar]
- 44.Lechevallier E, Andre M, Barriol D, et al. Fine-needle percutaneous biopsy of renal masses with helical CT guidance. Radiology. 2000;216(2):506–510. doi: 10.1148/radiology.216.2.r00au01506. [DOI] [PubMed] [Google Scholar]
- 45.Blumenfeld AJ, Guru K, Fuchs GJ, Kim HL. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology. 2010;76(3):610–613. doi: 10.1016/j.urology.2009.09.095. [DOI] [PubMed] [Google Scholar]
- 46.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60(3):578–584. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Pal SK, Kortylewski M, Yu H, Figlin RA. Breaking through a plateau in renal cell carcinoma therapeutics: development and incorporation of biomarkers. Mol Cancer Ther. 2010;9(12):3115–3125. doi: 10.1158/1535-7163.MCT-10-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58(3):398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 49.Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009;7(6):618–630. doi: 10.6004/jnccn.2009.0043. [DOI] [PubMed] [Google Scholar]
- 50.Vikram R, Caslino DD, Remer EM, et al. ACR appropriateness criteria: renal cell carcinoma staging. [Accessed October 23, 2012]. Available from: http://www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/RenalCellCarcinomaStaging.pdf.1995. Last reviewed 2011.
- 51.Graser A, Becker CR, Staehler M, et al. Single-phase dual-energy CT allows for characterization of renal masses as benign or malignant. Invest Radiol. 2010;45(7):399–405. doi: 10.1097/RLI.0b013e3181e33189. [DOI] [PubMed] [Google Scholar]
- 52.Gilet AG, Kang SK, Kim D, Chandarana H. Advanced renal mass imaging: diffusion and perfusion MRI. Curr Urol Rep. 2012;13(1):93–98. doi: 10.1007/s11934-011-0227-8. [DOI] [PubMed] [Google Scholar]
- 53.Taouli B, Thakur RK, Mannelli L, et al. Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology. 2009;251(2):398–407. doi: 10.1148/radiol.2512080880. [DOI] [PubMed] [Google Scholar]
- 54.Sun MR, Ngo L, Genega EM, et al. Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes – correlation with pathologic findings. Radiology. 2009;250(3):793–802. doi: 10.1148/radiol.2503080995. [DOI] [PubMed] [Google Scholar]
- 55.Smaldone MC, Chen DY, Uzzo RG, Yu M. Molecular imaging of the small renal mass. Urol Oncol. 2011;29(6):589–592. doi: 10.1016/j.urolonc.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Weber WA, Grosu AL, Czernin J. Technology Insight: advances in molecular imaging and an appraisal of PET/CT scanning. Nat Clin Pract Oncol. 2008;5(3):160–170. doi: 10.1038/ncponc1041. [DOI] [PubMed] [Google Scholar]
- 57.Hicks RJ, Ware RE, Lau EW. PET/CT: will it change the way that we use CT in cancer imaging? Cancer Imaging. 2006;6:S52–S62. doi: 10.1102/1470-7330.2006.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aide N, Cappele O, Bottet P, et al. Efficiency of [(18)F]FDG PET in characterising renal cancer and detecting distant metastases: a comparison with CT. Eur J Nucl Med Mol Imaging. 2003;30(9):1236–1245. doi: 10.1007/s00259-003-1211-4. [DOI] [PubMed] [Google Scholar]
- 59.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24(35):5601–5608. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 60.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 61.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vercellino L, Bousquet G, Baillet G, et al. 18F-FDG PET/CT imaging for an early assessment of response to sunitinib in metastatic renal carcinoma: preliminary study. Cancer Biother Radiopharm. 2009;24(1):137–144. doi: 10.1089/cbr.2008.0527. [DOI] [PubMed] [Google Scholar]
- 63.Ueno D, Yao M, Tateishi U, et al. Early assessment by FDG-PET/CT of patients with advanced renal cell carcinoma treated with tyrosine kinase inhibitors is predictive of disease course. BMC Cancer. 2012;12(1):162. doi: 10.1186/1471-2407-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kayani I, Avril N, Bomanji J, et al. Sequential FDG-PET/CT as a biomarker of response to Sunitinib in metastatic clear cell renal cancer. Clin Cancer Res. 2011;17(18):6021–6028. doi: 10.1158/1078-0432.CCR-10-3309. [DOI] [PubMed] [Google Scholar]
- 65.Harrison MR, George DJ. Better late than early: FDG-PET imaging in metastatic renal cell carcinoma. Clin Cancer Res. 2011;17(18):5841–5843. doi: 10.1158/1078-0432.CCR-11-1768. [DOI] [PubMed] [Google Scholar]
- 66.Oyama N, Okazawa H, Kusukawa N, et al. 11C-Acetate PET imaging for renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2009;36(3):422–427. doi: 10.1007/s00259-008-0981-0. [DOI] [PubMed] [Google Scholar]
- 67.Ozülker T, Ozülker F, Ozbek E, Ozpaçaci T. A prospective diagnostic accuracy study of F-18 fluorodeoxyglucose-positron emission tomography/computed tomography in the evaluation of indeterminate renal masses. Nucl Med Commun. 2011;32(4):265–272. doi: 10.1097/MNM.0b013e3283442e3b. [DOI] [PubMed] [Google Scholar]
- 68.Lawrentschuk N, Poon AM, Scott AM. Fluorine-18 fluorothymidine: a new positron emission radioisotope for renal tumors. Clin Nucl Med. 2006;31(12):788–789. doi: 10.1097/01.rlu.0000247310.05362.83. [DOI] [PubMed] [Google Scholar]
- 69.Lawrentschuk N, Poon AM, Foo SS, et al. Assessing regional hypoxia in human renal tumours using 18F-fluoromisonidazole positron emission tomography. BJU Int. 2005;96(4):540–546. doi: 10.1111/j.1464-410X.2005.05681.x. [DOI] [PubMed] [Google Scholar]
- 70.van Dongen GA, Poot AJ, Vugts DJ. PET imaging with radiolabeled antibodies and tyrosine kinase inhibitors: immuno-PET and TKI-PET. Tumour Biol. 2012;33(3):607–615. doi: 10.1007/s13277-012-0316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tolmachev V, Stone-Elander S. Radiolabelled proteins for positron emission tomography: pros and cons of labelling methods. Biochim Biophys Acta. 2010;1800(5):487–510. doi: 10.1016/j.bbagen.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 72.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40(3):167–181. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, et al. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38(4):489–494. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- 74.Oosterwijk E, De Weijert M, Van Bokhoven A, Brakenhoff RH, Peelen WP, Debruyne FMJ. Molecular characterization of the renal cell carcinoma-associated antigen G250. Proc Amer Assoc Cancer Res. 1996;37:461. [Google Scholar]
- 75.van Dijk J, Uemura H, Beniers AJ, et al. Therapeutic effects of monoclonal antibody G250, interferons and tumor necrosis factor, in mice with renal-cell carcinoma xenografts. Int J Cancer. 1994;56(2):262–268. doi: 10.1002/ijc.2910560220. [DOI] [PubMed] [Google Scholar]
- 76.Uemura H, Beniers AJ, Okajima E, Debruyne FM, Oosterwijk E. Vaccination with anti-idiotype antibodies mimicking a renal cell carcinoma-associated antigen induces tumor immunity. Int J Cancer. 1994;58(4):555–561. doi: 10.1002/ijc.2910580418. [DOI] [PubMed] [Google Scholar]
- 77.Uemura H, Okajima E, Debruyne FM, Oosterwijk E. Internal image anti-idiotype antibodies related to renal-cell carcinoma-associated antigen G250. Int J Cancer. 1994;56(4):609–614. doi: 10.1002/ijc.2910560424. [DOI] [PubMed] [Google Scholar]
- 78.Divgi CR, Bander NH, Scott AM, et al. Phase I/II radioimmunotherapy trial with iodine-131-labeled monoclonal antibody G250 in metastatic renal cell carcinoma. Clin Cancer Res. 1998;4(11):2729–2739. [PubMed] [Google Scholar]
- 79.Steffens MG, Boerman OC, Oosterwijk-Wakka JC, et al. Targeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250. J Clin Oncol. 1997;15(4):1529–1537. doi: 10.1200/JCO.1997.15.4.1529. [DOI] [PubMed] [Google Scholar]
- 80.Lam JS, Pantuck AJ, Belldegrun AS, Figlin RA. G250: a carbonic anhydrase IX monoclonal antibody. Curr Oncol Rep. 2005;7(2):109–115. doi: 10.1007/s11912-005-0036-7. [DOI] [PubMed] [Google Scholar]
- 81.Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol. 2010;58(1):75–83. doi: 10.1016/j.eururo.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Smaldone MC, Maranchie JK. Clinical implications of hypoxia inducible factor in renal cell carcinoma. Urol Oncol. 2009;27(3):238–245. doi: 10.1016/j.urolonc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158(3):905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Potter C, Harris AL. Hypoxia inducible carbonic anhydrase IX, marker of tumour hypoxia, survival pathway and therapy target. Cell Cycle. 2004;3(2):164–167. [PubMed] [Google Scholar]
- 85.Tostain J, Li G, Gentil-Perret A, Gigante M. Carbonic anhydrase 9 in clear cell renal cell carcinoma: a marker for diagnosis, prognosis and treatment. Eur J Cancer. 2010;46(18):3141–3148. doi: 10.1016/j.ejca.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 86.Sandlund J, Oosterwijk E, Grankvist K, Oosterwijk-Wakka J, Ljungberg B, Rasmuson T. Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int. 2007;100(3):556–560. doi: 10.1111/j.1464-410X.2007.07006.x. [DOI] [PubMed] [Google Scholar]
- 87.Patard JJ, Fergelot P, Karakiewicz PI, et al. Low CAIX expression and absence of VHL gene mutation are associated with tumor aggressiveness and poor survival of clear cell renal cell carcinoma. Int J Cancer. 2008;123(2):395–400. doi: 10.1002/ijc.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Martino M, Klatte T, Seligson DB, et al. CA9 gene: single nucleotide polymorphism predicts metastatic renal cell carcinoma prognosis. J Urol. 2009;182(2):728–734. doi: 10.1016/j.juro.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 89.Kim HL, Seligson D, Liu X, et al. Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol. 2005;173(5):1496–1501. doi: 10.1097/01.ju.0000154351.37249.f0. [DOI] [PubMed] [Google Scholar]
- 90.Kim HL, Seligson D, Liu X, et al. Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res. 2004;10(16):5464–5471. doi: 10.1158/1078-0432.CCR-04-0488. [DOI] [PubMed] [Google Scholar]
- 91.Oosterwijk E, Bander NH, Divgi CR, et al. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol. 1993;11(4):738–750. doi: 10.1200/JCO.1993.11.4.738. [DOI] [PubMed] [Google Scholar]
- 92.Brouwers AH, Dorr U, Lang O, et al. 131 I-cG250 monoclonal antibody immunoscintigraphy versus [18 F]FDG-PET imaging in patients with metastatic renal cell carcinoma: a comparative study. Nucl Med Commun. 2002;23(3):229–236. doi: 10.1097/00006231-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 93.Brouwers AH, Buijs WC, Mulders PF, et al. Radioimmunotherapy with [131I]cG250 in patients with metastasized renal cell cancer: dosimetric analysis and immunologic response. Clin Cancer Res. 2005;11(19 Pt 2):7178s–7186s. doi: 10.1158/1078-0432.CCR-1004-0010. [DOI] [PubMed] [Google Scholar]
- 94.Brouwers AH, Mulders PF, de Mulder PH, et al. Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. J Clin Oncol. 2005;23(27):6540–6548. doi: 10.1200/JCO.2005.07.732. [DOI] [PubMed] [Google Scholar]
- 95.Brouwers AH, Buijs WC, Oosterwijk E, et al. Targeting of metastatic renal cell carcinoma with the chimeric monoclonal antibody G250 labeled with (131)I or (111)In: an intrapatient comparison. Clin Cancer Res. 2003;9(10 Pt 2):3953S–3960S. [PubMed] [Google Scholar]
- 96.Bleumer I, Knuth A, Oosterwijk E, et al. A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer. 2004;90(5):985–990. doi: 10.1038/sj.bjc.6601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davis ID, Wiseman GA, Lee FT, et al. A phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinoma. Cancer Immun. 2007;7:13. [PMC free article] [PubMed] [Google Scholar]
- 98.Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC, et al. A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin- 2 pulsing scheme for advanced renal cell carcinoma. J Urol. 2006;175(1):57–62. doi: 10.1016/S0022-5347(05)00040-6. [DOI] [PubMed] [Google Scholar]
- 99.Steffens MG, Boerman OC, de Mulder PH, et al. Phase I radioimmunotherapy of metastatic renal cell carcinoma with 131I-labeled chimeric monoclonal antibody G250. Clin Cancer Res. 1999;5(Suppl 10):3268s–3274s. [PubMed] [Google Scholar]
- 100.Divgi CR, O’Donoghue JA, Welt S, et al. Phase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancer. J Nucl Med. 2004;45(8):1412–1421. [PubMed] [Google Scholar]
- 101.Siebels M, Rohrmann K, Oberneder R, et al. A clinical phase I/II trial with the monoclonal antibody cG250 (Rencarex) and interferon- alpha-2a in metastatic renal cell carcinoma patients. World J Urol. 2011;29(1):121–126. doi: 10.1007/s00345-010-0570-2. [DOI] [PubMed] [Google Scholar]
- 102.Bauer S, Oosterwijk-Wakka JC, Adrian N, et al. Targeted therapy of renal cell carcinoma: synergistic activity of cG250-TNF and IFNg. Int J Cancer. 2009;125(1):115–123. doi: 10.1002/ijc.24359. [DOI] [PubMed] [Google Scholar]
- 103.Bleumer I, Tiemessen DM, Oosterwijk-Wakka JC, et al. Preliminary analysis of patients with progressive renal cell carcinoma vaccinated with CA9-peptide-pulsed mature dendritic cells. J Immunother. 2007;30(1):116–122. doi: 10.1097/01.cji.0000211318.22902.ec. [DOI] [PubMed] [Google Scholar]
- 104.Uemura H, Fujimoto K, Tanaka M, et al. A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006;12(6):1768–1775. doi: 10.1158/1078-0432.CCR-05-2253. [DOI] [PubMed] [Google Scholar]
- 105.Pelosi E, Messa C, Sironi S, et al. Value of integrated PET/CT for lesion localisation in cancer patients: a comparative study. Eur J Nucl Med Mol Imaging. 2004;31(7):932–939. doi: 10.1007/s00259-004-1483-3. [DOI] [PubMed] [Google Scholar]
- 106.Brouwers A, Verel I, Van Eerd J, et al. PET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude rats. Cancer Biother Radiopharm. 2004;19(2):155–163. doi: 10.1089/108497804323071922. [DOI] [PubMed] [Google Scholar]
- 107.Verel I, Visser GW, Boerman OC, et al. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother Radiopharm. 2003;18(4):655–661. doi: 10.1089/108497803322287745. [DOI] [PubMed] [Google Scholar]
- 108.Uzzo RG, Russo P, Chen D, et al. The multicenter phase III Redect trial: a comparative study of 124 I-girentuximab-PET/CT versus diagnostic CT for the pre-operative diagnosis of clear cell renal cell carcinoma (ccRCC) [late-breaking abstract]. AUA Annual Meeting; May 29–June 3 2010; San Francisco, CA, USA. [Google Scholar]
- 109.Russo P, Jang TL, Pettus JA, et al. Survival rates after resection for localized kidney cancer: 1989 to 2004. Cancer. 2008;113(1):84–96. doi: 10.1002/cncr.23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brouwers AH, Mulders PF, Oyen WJ. Carbonic anhydrase IX expression in clear cell renal cell carcinoma and normal tissues: experiences from (radio) immunotherapy. J Clin Oncol. 2008;26(22):3808–3309. doi: 10.1200/JCO.2008.17.6073. author reply 11–12. [DOI] [PubMed] [Google Scholar]
- 111.Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11(1):71–77. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]