Abstract
Objectives
To define age-related geometric changes of the aortic arch and determine their relationship to central aortic stiffness and left ventricular remodeling.
Background
The proximal aorta has been shown to thicken, enlarge in diameter and lengthen with aging in humans. However, no systematic study has described age-related longitudinal and transversal remodeling of the aortic arch and their relationship with left ventricular mass and remodeling.
Methods
We studied 100 subjects (55 women, 45 men, average age: 46±16 years) free of overt cardiovascular disease using magnetic resonance imaging to determine aortic arch geometry (length, diameters, height, width and curvature), aortic arch function (local aortic distensibility and arch pulse wave velocity PWV) and left ventricular volumes and mass. Radial tonometry was used to calculate central blood pressure.
Results
Aortic diameters and arch length increased significantly with age. The ascending aorta increased most with age leading to aortic arch widening and decreased curvature. These geometric changes of the aortic arch were significantly related to decreased ascending aortic distensibility, increased aortic arch PWV (p<0.001) and to increased central blood pressures (p<0.001). Increased ascending aortic diameter, lengthening and decreased curvature of the aortic arch (unfolding) were all significantly associated with increased LV mass and concentric remodeling independently of age, gender, body size and central blood pressure (p<0.01).
Conclusions
Age-related unfolding of the aortic arch is related to increased proximal aortic stiffness in individuals without cardiovascular disease and associated with increased LV mass and mass-to-volume ratio independent of age, body size, central pressure and cardiovascular risk factors.
Keywords: magnetic resonance imaging, aortic geometry, aging, elasticity, left ventricular remodeling
Introduction
Aging, in a complex interplay with associated and aggravating factors such as disease, genetics and environmental factors, contributes to metabolic, structural and functional alterations of both large conduit arteries and microvessels (1, 2). Arterial stiffness is now recognized as an independent measure of cardiovascular risk beyond traditional risk factors (3–5). Stiffening of the proximal aorta has been shown to be strongly related to aging and to be one of the earliest manifestations of vascular aging in otherwise healthy humans (6). Alterations of the proximal aorta with age include structural and functional changes of the aortic wall and aortic pressure changes potentially leading to geometric and functional changes of the aortic arch. Ascending aortic diameter enlargement and lengthening have been described with advancing age (7) and correlated with increased global arterial stiffening measured as carotid to femoral pulse wave velocity by tonometry (8, 9). More recently, measures of proximal aortic function by MRI have been proposed and validated (6, 10, 11). MRI allows direct measurement of regional stiffness in the aortic arch (pulse wave velocity) in addition to local function such as distensibility in the ascending and descending aorta. Prior studies have shown the relationship between altered aortic arch geometry, increased aortic stiffness and sustained high blood pressure in adult patients with aortic coarctation (12). However, the comprehensive age-related changes in aortic arch geometry in a healthy population have not been described, except for a recent study that demonstrated age-related overall lengthening of the aorta without investigation of alterations in aortic diameters or aortic arch geometry (13). In this regard, alterations in aortic arch morphology may play an important role in explaining the age-related increase in aortic arch stiffness and central pulse pressure seen in asymptomatic older individuals (14). Changes in left ventricular mass and concentric remodeling have also been related to aging and are associated with poor cardiovascular outcome in large multiethnic general population studies (15) but the relationship between aortic and ventricular morphology in relation to age has not yet been described. MRI together with central pressures calculated with arterial tonometry can provide a comprehensive analysis of both aortic and ventricular morphology and function non-invasively.
In this prospective cross-sectional study, we sought to investigate the interaction between age and aortic arch geometry, including lengthening, widening, and altered curvature, measures of aortic arch stiffness and left ventricular mass and geometry.
Methods
Study subjects
We studied 108 consecutive subjects. All subjects were informed about the study protocol and provided written consent. The procedures followed were in accordance with institutional guidelines and the Declaration of Helsinki and the study was approved by The Johns Hopkins University Ethics Review Board. This was a general population study sample from the Baltimore community. Subjects enrolled in the study were asked questions from a standardized questionnaire, including medication regimen. Inclusion criteria were in the absence of contraindications to MRI: age>18 years, absence of acute or chronic disease including diabetes, no personal history or symptoms of cardiac disease, normal physical examination and normal electrocardiogram. Hypertension was defined as diastolic blood pressure ≥90 mmHg, systolic blood pressure ≥140 mmHg, or receiving treatment for hypertension. Hyperlipidemic patients were defined as either participants with known abnormal lipid levels or receiving lipid-lowering therapy. None of the screened subjects presented a family history of aortic disease, sudden death, Marfan syndrome or connective tissue disease. Height and weight were measured and BMI was used as a measure of global adiposity.
Image Acquisition and Analysis
All images were acquired on a 3.0T scanner (Trio Tim, Siemens) using ECG gating and breath-holding with a 6-element thoracic coil for radiofrequency signal detection.
Assessment of LV Function and Mass by MRI
To measure end-diastolic and end-systolic LV volumes and end-diastolic LV mass, endocardial and epicardial borders were traced semi automatically on 8 to 10 short axis cine MRI slices covering the entire LV using QMass (MEDIS, The Netherlands). End-diastolic LV mass to volume ratio was calculated (M/V) and used as a measure of concentric remodeling.
Aortic Geometry and Function Measurements
To visualize the aorta, we acquired 4 sagittal oblique views of the aortic arch using a black-blood spin echo sequence (slice thickness:6mm, no gap, matrix:256×256). A gradient echo pulse sequence with through-plane velocity encoding simultaneously providing the velocities in the ascending and descending aorta was applied perpendicular to the aorta at the level of pulmonary artery bifurcation. Maximal velocity encoding was 150cm/s, slice thickness:6mm, matrix:192×192 and temporal resolution:20ms. The same slice location was used to acquire an aortic cine using a fast retrospectively gated gradient echo sequence (slice thickness:6mm, matrix:256×256, temporal resolution:20ms) followed by an acquisition perpendicular to the diaphragmatic descending thoracic aorta.
The contours of the ascending, proximal and distal (diaphragmatic) descending aorta were automatically traced for all phases of the cardiac cycle on both the modulus images of the phase contrast acquisition for flow analysis and on the cine images for aortic area analysis using the ARTFUN software (INSERM U678) (16, 17).
The maximal (Amax) and minimal (Amin) aortic lumen areas were used to calculate average aortic diameters of the ascending and proximal and distal descending aorta. Relative change in area (aortic strain) defined as AS= (Amax-Amin)/Amin was used to calculate aortic distensibility in each subject as follows: distensibility=AS/cPP where cPP is the central pulse pressure (PP) obtained by tonometry. Aortic arch pulse wave velocity (PWV) was calculated by using the transit time of the flow curves and the distance between the ascending and proximal descending aortic locations of the phase-contrast acquisition as previsouly described (6).
The length of the aortic arch traveled by the flow wave (L1) was measured as the centerline of the aorta. Eight to 10 control points were manually placed on the central black blood views of the aortic arch in the center of the vessel and their 3D coordinates recorded (Figure 1). The first and last points were placed at the center of respectively the ascending and descending aorta and in the plane used for the velocity acquisition. We then performed a spline interpolation to our manual points to construct a 3D vessel centerline curve and calculated the following parameters: 1. Aortic arch width (W): given by the distance between the center of the ascending and descending aorta 2. Aortic arch height (H): defined as the length of the orthogonal projection of the curve’s inflection point at the top of the arch on the width of the aortic arch. 3. We calculated the H/W ratio for the aortic arch defined as the height (H) divided by the width (W). 4. Curvature: following Wood et al. (18) we calculated the average aortic arch curvature (AC) expressed in mm−1 which is an estimate of the global tortuosity of the aortic arch. See appendix for details. In addition, we measured the centerline vascular distance (L2) between the proximal and distal descending aortic acquisition planes (Figure 1).
Figure 1. Aortic Arch Geometry assessment with MRI.
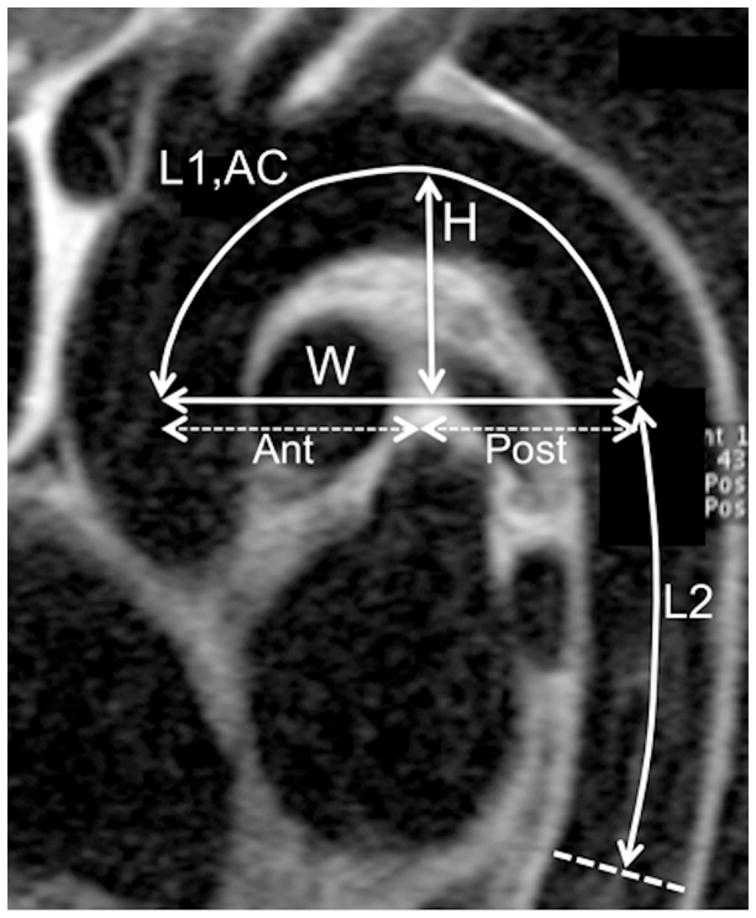
Sagittal oblique view of the thoracic aorta using a spin echo black blood MRI acquisition illustrating aortic measurements: L1: length of the aortic arch, AC: average arch curvature, H: arch height, W: arch width, Ant: anterior arch width, Post: posterior arch width. L2: length of the descending aorta.
Tonometry Data Acquisition and Analysis
Tonometry was performed immediately after MRI at rest in supine position in a quiet, temperature-controlled room. We used a commercially available device (VP-2000, Colin Corporation, Komaki, Japan) customized to output all physiological signals, including electrocardiogram, phonocardiogram, oscillometric signals and tonometric signals. Brachial systolic and diastolic pressures were the averages of 4 oscillometric measurements (2 on each side) and were used to calculate mean brachial pressure. All signals were digitized simultaneously at a sampling frequency of 250Hz for off-line analysis. Right radial artery waveforms were recorded for 30 seconds. Then, central aortic pressure waveforms were reconstructed for each subject from the radial waveforms using a generalized transfer function as in (19).
Statistical Analysis
Baseline characteristics are provided as means±SD for continuous variables and percentages±SD for discrete variables. To present an exploratory analysis for the trends of the different arterial parameters over age, we grouped the subjects into six age strata of 10 years, and calculated the conditional means±SD of the arterial parameters given each age group. Age group sample size: 20–29 years: n=20, 30–39: n=16, 40–49:n=26, 50–59:n=15, 60–69:n=13, >70:n=10. The general distribution of values of the arterial parameters across age groups and quartiles of aortic stiffness were evaluated using using the analysis of variance ANOVA F-test at 5% significant level.
Potential covariates with clinical relevance, such as gender and BMI, were selected by examining their significance in univariate and multivariate models and the stepwise variable selection procedures. Univariate correlations between arterial measures was reported using Pearson correlation coefficients. When LV mass and mass-to-volume ratio are taken separately as continuous random variables, the relationship between age, body size, blood pressure and other cardiovascular risk factors and the arterial parameters were studied using multivariate regression models. All reported p-values are two-sided and a p-value of less than 0.05 is used to indicate statistical significance. Analysis was done with STATA 10IC.
Results
Study subjects
Of the 108 participants enrolled, 6 did not complete MRI due to claustrophobia and 2 failed to complete the protocol leaving 100 studies (55 women, 45 men, mean age:46±16 years, range:20–84) for analysis. Fifty-seven subjects free of cardiovascular risk factors and 43 subjects having at least one cardiovascular risk factor at the exclusion of diabetes were studied of which 33 had hypertension, 8 were current smokers and 18 had hypercholesterolemia. Of hypertensive subjects 24 (73%) had antihypetensive medication of whom 16 (67%) received diuretics, 13 (54%) an ACE-inhibitor or angiotensin receptor blocker, 8 (33%) a beta-blocker.
Subject characteristics are summarized in Table 1. Men were, on average, significantly taller than women and had an increased aortic arch height, width and length and a lower average arch curvature as well as slightly higher descending aortic diameters and length compared to women. However, peripheral and central blood pressures and aortic stiffness (local aortic distensibilities and aortic arch PWV) were similar in both genders. None of the significant unadjusted differences between men and women persisted after adjustment of aortic geometry measures for BSA and BMI except for aortic arch curvature adjusted for BSA (p=0.001), indicating that body proportions play a significant role in explaining the apparent differences by gender.
Table 1.
Patient Characteristics
| Patient Characteristics | Women ( n=55) | Men (n=45) | (p) |
|---|---|---|---|
| Age, years | 48±17 | 45±15 | 0.24 |
| Height, cm | 165±6 | 175±8 | <0.01 |
| Weight, kg | 72±18 | 80±13 | 0.01 |
| BMI | 26±6 | 26±5 | 0.89 |
| Heart Rate, bpm | 66±9 | 61±11 | 0.02 |
| Brachial Pressures, mmHg | |||
| SBP | 122±18 | 124±20 | 0.46 |
| DBP | 69±10 | 73±13 | 0.08 |
| PP | 52±12 | 51±11 | 0.59 |
| Central Pressures, mmHg | |||
| SBP | 113±20 | 113±23 | 0.92 |
| DBP | 68±10 | 72±14 | 0.09 |
| PP | 45±15 | 41±14 | 0.21 |
| Ascending Aorta | |||
| Diameter, mm | 30± | 31±4 | 0.24 |
| Strain, % | 19±12 | 18±12 | 0.86 |
| Distensibility, kPa−1.10−3 | 38±31 | 38±27 | 0.96 |
| Proximal Descending Aorta | |||
| Diameter, mm | 22±3 | 24±3 | <0.01 |
| Strain, % | 23±12 | 22±10 | 0.94 |
| Distensibility, kPa−1.10−3 | 44±30 | 45±24 | 0.84 |
| Distal Descending Aorta | |||
| Diameter, mm | 20±2 | 21±3 | 0.02 |
| Strain, % | 37±19 | 44±18 | 0.06 |
| Distensibility, kPa−1.10−3 | 73±47 | 88±38 | 0.09 |
| Aortic Arch Width, mm | 66±10 | 72±11 | <0.01 |
| Aortic Arch Height, mm | 37±6 | 40±7 | 0.01 |
| H/W | 0.56±0.09 | 0.56±0.09 | 0.99 |
| Aortic Arch Length (L1), mm | 110±15 | 122±21 | <0.01 |
| Descending Aorta Length (L2), mm | 134±16 | 143±15 | <0.01 |
| Average aortic Arch Curvature, mm−1 | 0.032±0.004 | 0.029±0.004 | <0.01 |
| Aortic Arch PWV, m/s | 6.5±4.0 | 5.7±2.4 | 0.24 |
| LV mass, g | 112±28 | 150±34 | <0.01 |
| LV mass index, g/m² | 62±12 | 76±16 | <0.01 |
| LV M/V ratio, g/ml | 0.94±0.2 | 1.06±0.2 | <0.01 |
Note: BMI: Body Mass Index, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, PP: Pulse Pressure, H/W: aortic arch height over width ratio, PWV: Pulse Wave Velocity, LV: left ventricle, M/V: end-diastolic mass over volume ratio.
Age, Body Size and Aortic Arch Geometry
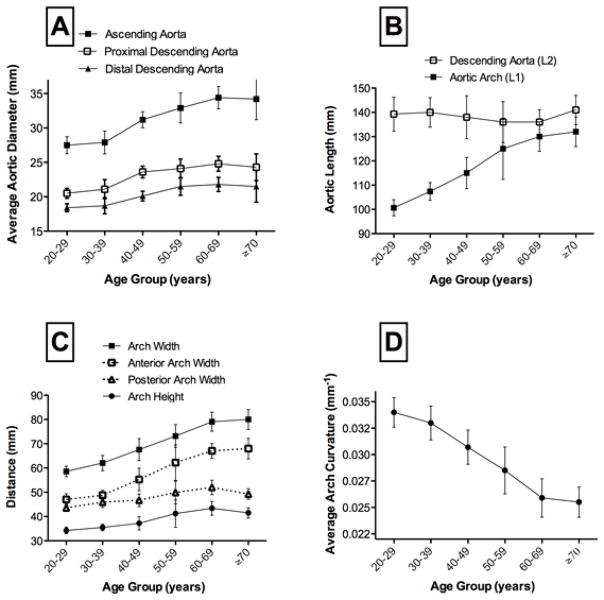
Relative adult lifetime changes of aortic dimensions are summarized on Table 2 and Figure 2. Average aortic diameters increased with age (p<0.0001) but with important regional differences. Indeed, the magnitude of diameter increase of the ascending aorta was slightly greater than that of the proximal and distal descending aorta (Figure 2A) with respective increases of 21% (27.5 to 33.2mm), 19% (20.5 to 24.3mm) and 17% (18.3 to 21.5mm) from the 2d to 7th decade. On average, the diameter of the ascending aorta increased by 0.11mm/year. Moreover, the length of the aortic arch (L1) increased significantly with age in our study (Figure2B) with an average increase of 30% (100.4 to 130.9mm) from the 2d to 7th decade and an increase of 6mm per 10 years (p<0.0001). In contrast, descending aortic length (L2) did not change significantly (p=0.27). The overall proportions of the arch changed with aging because the increase in arch width: +34% (59±5mm to 79±9mm) significantly exceeded the increase in arch height: +21% (34±3mm to 42±5mm) from the 2d to the 7th decade (Figure 2C). Consequently, the H/W ratio decreased with age. Furthermore, the observed widening of the aortic arch was not symmetric with a notably greater increase of the anterior compared to the posterior portion of the arch (Figure 2C). Hence, most of the age associated widening of the arch was related to an elongation of the anterior portion of the arch. The average curvature of the arch (AC) decreased progressively with aging up to the sixth decade (Figure 2D). Importantly, there were no significant associations between measures of aortic arch geometry and body height. Conversely, increased body weight was associated with increased aortic arch width (r=0.34, p<0.001) and increased aortic diameters (ascending aorta r=0.24, p=0.01) as well as decreased aortic arch curvature (r=−0.29, p=0.003). Overall, these correlations were not very strong although negative correlation between the H/W ratio and body weight was relatively strong (r=−0.41, p<0.001).
Table 2.
Relative change in measures of Aortic Geometry with Age: comparison of young (<30 years) and older subjects (>70 years)
| Age<30 years | Age>70 years | Average Annual Change | Relative change | |
|---|---|---|---|---|
| Ascending Aorta Diameter, mm | 27.5±2.8 | 33.2±4.3 | +0.11mm/year | +21% |
| Aortic Arch Length, mm | 100.4±7.1 | 130.9±13.9 | +0.60mm/year | +30% |
| Aortic Arch Width, mm | 58.6±4.7 | 78.5±8.7 | +0.40mm/year | +34% |
| Aortic Arch Height, mm | 34.3±2.9 | 41.5±4.5 | +0.14mm/year | +21% |
| Aortic Arch Curvature, mm−1 | 0.034±0.002 | 0.027±0.003 | −0.14.10−3 mm−1/year | −21% |
| Proximal Descending Aorta Diameter, mm | 20.5±1.6 | 24.3±2.7 | +0.08mm/year | +19% |
| Distal Descending Aorta Diameter, mm | 18.3±1.4 | 21.5±3.2 | +0.06mm/year | +17% |
| Descending Aorta Length, mm | 139.3±15.8 | 142.6±13.3 | +0.07mm/year | +2% |
Figure 2. Effect of Age on Measures of Aortic Geometry.
Relationship with age of : Panel A: ascending and descending proximal and distal aortic diameters. Panel B: aortic arch and descending aortic lengths. Panel C: breakdown of local aortic arch dimensions. Panel D: average aortic arch curvature. Age group sample size: 20–29 years: n=20, 30–39: n=16, 40–49:n=26, 50–59:n=15, 60–69:n=13, >70:n=10. Statistical significance for trend across age categories : *p<0.0001, †p<0.01, ‡p=0.09.
Relationships between Aortic Arch Geometry and Blood Pressure
We found significant correlations between measures of aortic arch geometry and both brachial and central blood pressures. More specifically, increased ascending aortic diameter and increased aortic arch length, width as well as decreased aortic arch curvature were highly correlated with increased central and brachial systolic, diastolic, mean and pulse pressures (p<0.001). Overall, the strongest correlations were found between increased ascending aortic diameter, increased aortic arch length, decreased curvature and systolic blood pressures (respectively: r=0.61, r=0.64, r=−0.71, p<0.001 for brachial SBP and r=0.62, r=0.65, r=−0.70, p<0.001 for central SBP). After adjustment for age, gender, body height and weight, average ascending aortic diameter (R2=0.53, p=0.003), arch length (R2=0.56, p<0.001) and arch curvature (R2=0.57, p<0.001) were all independent associates of central SBP. Similar independent associations were obtained between aortic geometry measures and brachial systolic blood pressure. These relationships remained significant after further adjustment for other risk factors such as hypertension, antihypertension medication, hypercholesterolemia and smoking. When both the brachial and central SBP were added in the multivariate regression models the two pressures were not independent and age remained the strongest associate of all aortic geometry measures.
Relationships between Aortic Arch Geometry and Stiffness
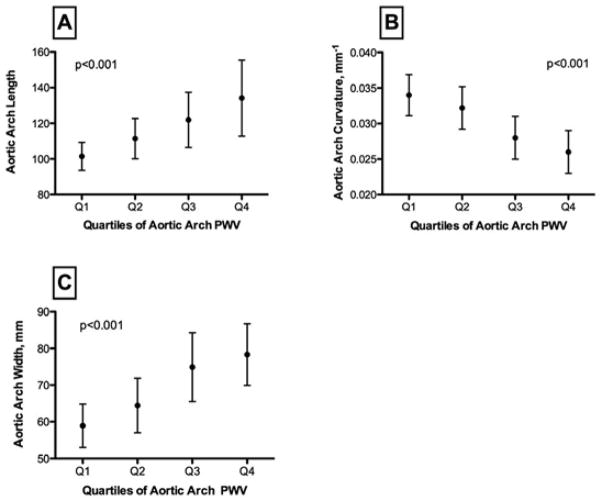
As shown on Figure 3, increased ascending aorta diameter was significantly associated with increased regional stiffness of the proximal aorta i.e. increased aortic arch PWV (r=0.56, p<0.001). Furthermore, increased length and width and decreased curvature of the aortic arch were strongly associated with increased local and regional aortic stiffness. In particular, decreased aortic arch curvature was related to respectively decreased aortic distensibility (r=0.65, p<0.001) and increased aortic arch PWV (r=−0.61, p<0.001), Figure 3. In multivariate analysis, after adjustment for age, gender, body height, weight and central SBP, we observed a significant association between increased arch PWV, decreased aortic arch curvature (p=0.01) and increased aortic arch length (p=0.03). These relationships remained significant after further adjustment for other risk factors such as hypertension, antihypertensive medication, hypercholesterolemia and smoking.
Figure 3. Relationship between Aortic Stiffness and Aortic Geometry.
Distribution of the following indices of aortic arch geometry by quartiles of aortic arch PWV: Panel A: aortic arch length (mm), Panel B: aortic arch curvature (mm−1), Panel C: aortic arch width (mm). p: significance level for ANOVA F-test.
Relationship between Aortic Geometry and LV Mass and M/V
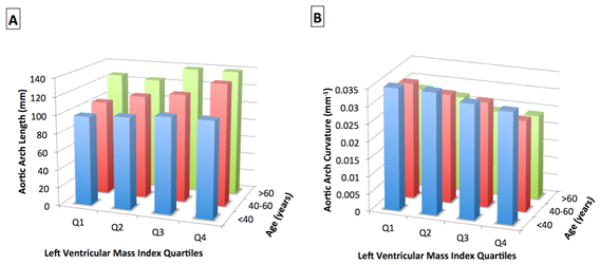
Univariate analysis showed significant correlations between increased aortic arch width, decreased aortic arch curvature and increased LV mass and mass to volume ratio. The aortic arch geometry parameter which most strongly correlated with LV mass and M/V was aortic arch curvature. Decreased arch curvature was significantly associated with increased LV mass (r=0.46, p<0.001) and M/V (r=0.41, p<0.001). The interrelationship between LV mass, aortic geometry and age is illustrated on Figure 4. Across age groups, we found a significant trend for subjects in the higher quartiles of LV mass to have significantly increased aortic arch length and decreased curvature, beyond the effect of age. Multivariate analysis (Table 3) showed that increased ascending and descending aortic diameter, increased aortic arch length, height and width and decreased arch curvature are related to increased LV mass independent of age, gender, body size and central SBP. The strongest aortic geometry associates of LV mass were ascending aortic diameter and aortic arch length and curvature. Furthermore, increased ascending aortic diameter, increased aortic arch length and decreased arch curvature were independently related to increased LV M/V ratio although significance levels were lower than in LV mass models. These results were obtained after adjustment for the presence of other cardiovascular risk factors such as hypertension, antihypertensive medication, smoking and hypercholesterolemia. The addition of antihypertensive medication as a dichotomous variable or the addition of all individual antihypertensive medications did not change the strength of the results concerning the relationship between aortic geometry, aortic stiffness and LV mass/remodeling. However, ACE-inhibitors and ARBs were independently associated with increased aortic diameter, arch length and aortic unfolding whereas no significant impact of other drugs was found. Furthermore, when both the brachial and central SBP were added in the multivariate regression models exploring relationships between aortic geometry and LV mass the two pressures were not independent.
Figure 4. Relationships of Aortic Geometry to Left Ventricular Mass Index and Age.
Relationship of aortic arch length (Panel A) and aortic arch curvature (Panel B) according to quartiles of LV mass index and to age group: <40, 40–60 and >60 years.
Table 3.
Relationships between Measures of Aortic Geometry and Left Ventricular Mass and Geometry Adjusted for Age, Gender, Height, Weight and Central Systolic Blood Pressure.
| LV mass (/10g) | LV M/V (/0.1) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| β | Individual (p) | Overall R2 (p) | β | Individual (p) | Overall R2 (p) | |
| Model A | 0.55 (<0.001) | 0.25 (<0.001) | ||||
| Age, years | −5±2 | 0.01 | 0.21 | |||
| Male Gender | <0.001 | 0.002 | ||||
| Height, cm | 2.0±3.8 | 0.61 | 0.32 | |||
| Weight, kg | 7.2±1.7 | <0.001 | 0.25 | |||
| Central SBP, mmHg | 8.0±1.7 | <0.001 | 0.11 | |||
|
| ||||||
| Model B | 0.64 (<0.001) | 0.28 (<0.001) | ||||
| Hypertension | 0.001 | 0.74 | ||||
| Hypercholesterolemia | 0.12 | 0.93 | ||||
| Current smoking | 0.03 | 0.06 | ||||
|
| ||||||
| Model C | ||||||
| Ascending Aorta Diameter, mm | 25±7.3 | 0.001 | 0.69 (<0.001) | 0.14±0.04 | 0.002 | 0.36 (<0.001) |
| Descending Aorta Diameter, mm | 34±12 | 0.006 | 0.68 (<0.001) | 0.23±0.07 | 0.002 | 0.36 (<0.001) |
| Aortic Arch Length, mm | 6.1±2.0 | 0.002 | 0.68 (<0.001) | 0.029±0.01 | 0.01 | 0.33 (<0.001) |
| Aortic Arch Width, mm | 10.8±4.0 | 0.008 | 0.67 (<0.001) | 0.06±0.02 | 0.02 | 0.33 (<0.001) |
| Aortic Arch Height, mm | 8.9±4.3 | 0.04 | 0.66 (<0.001) | 0.04±0.02 | 0.08 | 0.30 (<0.001) |
| Arch Curvature, ×100 | −21±6.5 | 0.002 | 0.68 (<0.001) | −11.6±5.3 | 0.03 | 0.32 (<0.001) |
Regression coefficient β is given for continuous variables for a change in LV Mass of 10 grams and change in M/V of 0.1 in respectively: 1) Model A: a base model including: age, gender, height, weight and central systolic blood pressure 2) Model B: a model including the variables of Model A and with the addition of conventional risk factors: hypertension, hypercholesterolemia and active smoking 3) Model C: individual models corresponding to Model B with the separate addition of each aortic measure. Overall model significance: R2, significance level: p<0.05.
Discussion
Our study demonstrates a close relationship between alterations in proximal aortic geometry, namely aortic unfolding (elongation and widening of the aortic arch) and increased left ventricular mass and concentric remodeling. Although mainly related to aging, this vascular-ventricular relationship remains significant after adjustment for age, gender, body size, central blood pressure and traditional cardiovascular risk factors. Secondly, we demonstrate a strong relationship between aortic unfolding and 1) increased central and brachial blood pressures and 2) altered local and regional aortic function (increased stiffness).
Age-related arterial function change is considered to be an important independent determinant of cardiovascular morbidity and mortality (4, 5, 20). The aorta is subject to constant pulsatile stress, so that the elastic components of the aortic media fragment and eventually break down to be partially replaced by mostly fibrotic non elastic tissue (21). These histologic processes lead to stiffening of the aortic wall and increased mean aortic blood pressure and finally to transverse dilation of the aorta. This mechanism of increased central arterial volume may initially compensate for stress-induced alteration of aortic function and elasticity but may also progressively lead over time to chronically increased LV afterload and promote LV hypertrophy and concentric remodeling (22). The increase in aortic diameter with age is well known and described (23) and has been classically related to prominent aortic knuckle and aortic unfolding on chest radiography. However, the longitudinal alterations have been much less studied largely because of difficult access to detailed imaging of the proximal aorta. A recent study showed a preferential age-related elongation of the ascending aorta using MRI in apparently healthy subjects and demonstrated the importance of measuring the central vascular distances when calculating the pulse wave velocity (13). Our study demonstrates that the age related longitudinal elongation of the aortic arch exceeds the transversal dilation process whereas the opposite is true for the descending aorta. This may be partially secondary to the relative fixation of the descending aorta to the spine by intercostal arteries and the horizontal portion of the aortic arch by the neck arteries in contrast to the free mediastinal space occupied by the ascending aorta from its anterior LV attachment up to the horizontal portion of the arch. We found a diameter increase in the ascending aorta of 1.1mm/decade which is consistent with the value of 0.96mm/decade reported by Hickson et al. (8). The length and diameter increases of the ascending aorta reported here were also predominant over changes in the descending aorta in their study. Taken together, these findings indeed suggest that the age-related dilation and lengthening hence augmentation of proximal aortic volume may help to offset wall stiffening and loss of distensibility by augmenting the storage capacity of systolic blood volume.
This study shows a close relationship between alterations in aortic geometry and decreased proximal aortic function, thus defining an age-related vascular phenotype. Furthermore we demonstrate an association between changes in aortic arch geometry and an increase in all components of central and brachial blood pressure, particularly systolic, mean and pulse pressures independently of age, gender and body size. This altered aortic phenotype combines transversal and longitudinal enlargement of the vessel, loss of the harmonious arch morphology and unfolding (decrease in average curvature), loss of arterial elasticity and elevation of regional pulse wave velocity. It is noteworthy that aortic arch width could be used as a surrogate and more simple measure of aortic unfolding and geometry alteration. We postulate that these structural aortic alterations are integrated markers summarizing, at the time they are measured, large artery damage but also ventricular remodeling over one’s past lifetime. While age is considered to be an important determinant of these arterial changes, its effects are clearly modulated by disease, exposure to traditional cardiovascular risk factors, explaining, along with genetic and other constitutional factors, the heterogeneity of measures of aortic stiffness in younger individuals.
LV hypertrophy has been shown to be a predictor of cardiovascular events and sudden death in large population studies (24, 25). More recently, increased LV mass and concentric remodeling measured by MRI have further been shown to be predictors of incident cardiovascular events (26). The prognostic role of concentric remodeling without hypertrophy has also been discussed (27–29). A recent study found increased M/V ratio to be the most representative feature of age-related LV changes because of differential changes in LV volumes and mass during the aging process in a large multiethnic cohort. M/V ratio was a stronger predictor of all cardiovascular events in younger individuals (<65 years) suggesting that subclinical concentric remodeling in younger subjects could indicate a higher risk (15). We have previsouly shown that alterations of aortic function beagn early in life before significant changes in aortic diameter in the absence of blood pressure modifications and in the absence of significant LV hypertrophy or remodeling (6). However, direct relationships between aortic stiffness and LV dysfunction have been reported suggesting that an early increase in arterial stiffness may lead to subclinical LV dysfunction (15, 30, 31). Since aortic diameter, arch length and curvature independently predicted LV mass and M/V in our study they might be sensitive markers of coupled subclinical vascular-ventricular alterations. Aortic morphology alterations may be among causal factors in the pathway leading to LV hypertrophy and concentric remodeling. However, the cross sectional design of our study does not allow us to prove time or causal relationships. Nevertheless, our results show that proximal aortic and LV remodeling are strongly coupled, independently from central and brachial blood pressure levels and more strongly related to vascular age than calendar age.
Limitations of our study also include the time difference, albeit short, between central pressure measurements and MRI acquisition as we could not perform the measurements inside the magnet. However, we tried to minimize this bias by numerous pressure acquisitions immediately after MRI in a similar environment. We estimate global tortuosity of the aortic arch defined as an average of local curvatures from 3D centerline points. Future works could measure local or segmental tortuosity of the thoracic aorta requiring very detailed centerline measurement using automated methods on 3D aortic acquisitions. Furthermore, the study sample does not allow to generalize results to define normal aortic arch values for an individual. Further studies with longitudinal design are necessary to determine the chain of causality in defining patterns of vascular-ventricular coupling during aging.
Clinical perspectives
Management strategy of aneurysms of the thoracic aorta mainly rely on diameters. However, we know that aortic dissection may occur in only moderately dilated aortas, below commonly used diameter thresholds warranting surgical treatment. Our study shows an average increase of aortic diameter of 7mm over 5 decades of life whereas arch length increased an average of 20mm during the same period. However, we do not know however clearly what the predictive value of aortic length or tortuosity could be for adverse aortic events. In this regard, a better understanding of the biomechanics of the aorta in vivo in humans using a non invasive imaging technique is a prerequisite to determine valuable new markers (morphology and function) of age and disease-related subclinical alterations that may be more sensitive to identify in future studies aortic phenotypes at high risk for potentially lethal aortic complications. Furthermore, novel measures of aortic morphology such as volume could help to determine potentially reversible aortic alterations early and prevent evolution toward LV hypertrophic remodeling and dysfunction.
Conclusions
Age-related alterations of aortic arch geometry, in particular aortic unfolding, are related to functional aortic alterations such as decreased aortic distensibility and augmented aortic arch PWV in individuals without overt cardiovascular disease. Furthermore, increased aortic arch length and decreased curvature are associated with increased left ventricular mass beyond calendar age, body size, central pressure and cardiovascular risk factors.
Supplementary Material
Acknowledgments
Funding Sources : Alban Redheuil received partial grant support from Fédération and Société Française de Cardiologie and Société Française de Radiologie.
The authors wish to thank Elzbieta Chamera, Rosalie Cosgriff and Alain DeCesare for their important contribution to data collection and participant management.
Abbreviations
- MRI
Magnetic Resonance Imaging
- LV
Left Ventricle
- ECG
Electrocardiogram
- AS
Aortic Strain
- PP
Pulse Pressure
- PWV
Pulse Wave Velocity
- SD
Standard Deviation
- SBP
Systolic Blood Pressure
- M/V
Left Ventricular End diastolic Mass to Volume Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a « set up » for vascular disease. Circulation. 2003;107 (1):139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15 (5):426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27 (21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Hwang S-J, Vasan RS, et al. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation. 2010;121 (4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness: A Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2010;55 (13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Redheuil A, Yu W-C, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55 (2):319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell J, Schwartz C. Arterial Disease. Philadelphia, PA: FA Davis; 1965. [Google Scholar]
- 8.Hickson SS, Butlin M, Graves M, et al. The Relationship of Age With Regional Aortic Stiffness and Diameter. JACC: Cardiovascular Imaging. 2010;3 (12):1247–1255. doi: 10.1016/j.jcmg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-Associated Elongation of the Ascending Aorta in Adults. JACC: Cardiovascular Imaging. 2008;1 (6):739–748. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Rogers WJ, Hu YL, Coast D, et al. Age-associated changes in regional aortic pulse wave velocity. J Am Coll Cardiol. 2001;38 (4):1123–1129. doi: 10.1016/s0735-1097(01)01504-2. [DOI] [PubMed] [Google Scholar]
- 11.Mohiaddin RH, Firmin DN, Longmore DB. Age-related changes of human aortic flow wave velocity measured noninvasively by magnetic resonance imaging. J Appl Physiol. 1993;74 (1):492–497. doi: 10.1152/jappl.1993.74.1.492. [DOI] [PubMed] [Google Scholar]
- 12.Ou P, Celermajer DS, Jolivet O, et al. Increased central aortic stiffness and left ventricular mass in normotensive young subjects after successful coarctation repair. Am Heart J. 2008;155 (1):187–193. doi: 10.1016/j.ahj.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1 (6):739–748. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 14.O’Rourke M, Farnsworth A, O’Rourke J. Aortic dimensions and stiffness in normal adults. JACC Cardiovasc Imaging. 2008;1 (6):749–751. doi: 10.1016/j.jcmg.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S, Fernandes VRS, Bluemke DA, McClelland RL, Kronmal RA, Lima JAC. Age-Related Left Ventricular Remodeling and Associated Risk for Cardiovascular Outcomes: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2 (3):191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herment A, Lefort M, Kachenoura N, et al. Automated estimation of aortic strain from steady-state free-precession and phase contrast MR images. Magn Reson Med. 2010 doi: 10.1002/mrm.22678. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 17.Herment A, Kachenoura N, Lefort M, et al. Automated segmentation of the aorta from phase contrast MR images: validation against expert tracing in healthy volunteers and in patients with a dilated aorta. J Magn Reson Imaging. 2010;31 (4):881–888. doi: 10.1002/jmri.22124. [DOI] [PubMed] [Google Scholar]
- 18.Wood NB, Zhao SZ, Zambanini A, et al. Curvature and tortuosity of the superficial femoral artery: a possible risk factor for peripheral arterial disease. J Appl Physiol. 2006;101 (5):1412–1418. doi: 10.1152/japplphysiol.00051.2006. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95 (7):1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. European Heart Journal. 2006;27 (21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 21.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50 (1):1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 22.O’Rourke M. Arterial stiffening and vascular/ventricular interaction. J Hum Hypertens. 1994;8 (Suppl 1):S9–15. [PubMed] [Google Scholar]
- 23.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45 (4):652–658. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 24.Levy D, Garrison R, Savage D, Kannel W, Castelli W. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322 (22):1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 25.Di Tullio MR, Zwas DR, Sacco RL, Sciacca RR, Homma S. Left ventricular mass and geometry and the risk of ischemic stroke. Stroke. 2003;34 (10):2380–2384. doi: 10.1161/01.STR.0000089680.77236.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52 (25):2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358 (13):1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 28.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25 (4):879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 29.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35 (3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 30.Kang S, Fan H-M, Li J, et al. Relationship of arterial stiffness and early mild diastolic heart failure in general middle and aged population. European Heart Journal. 2010;31 (22):2799–2807. doi: 10.1093/eurheartj/ehq296. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes VR, Polak JF, Cheng S, et al. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28 (1):194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.