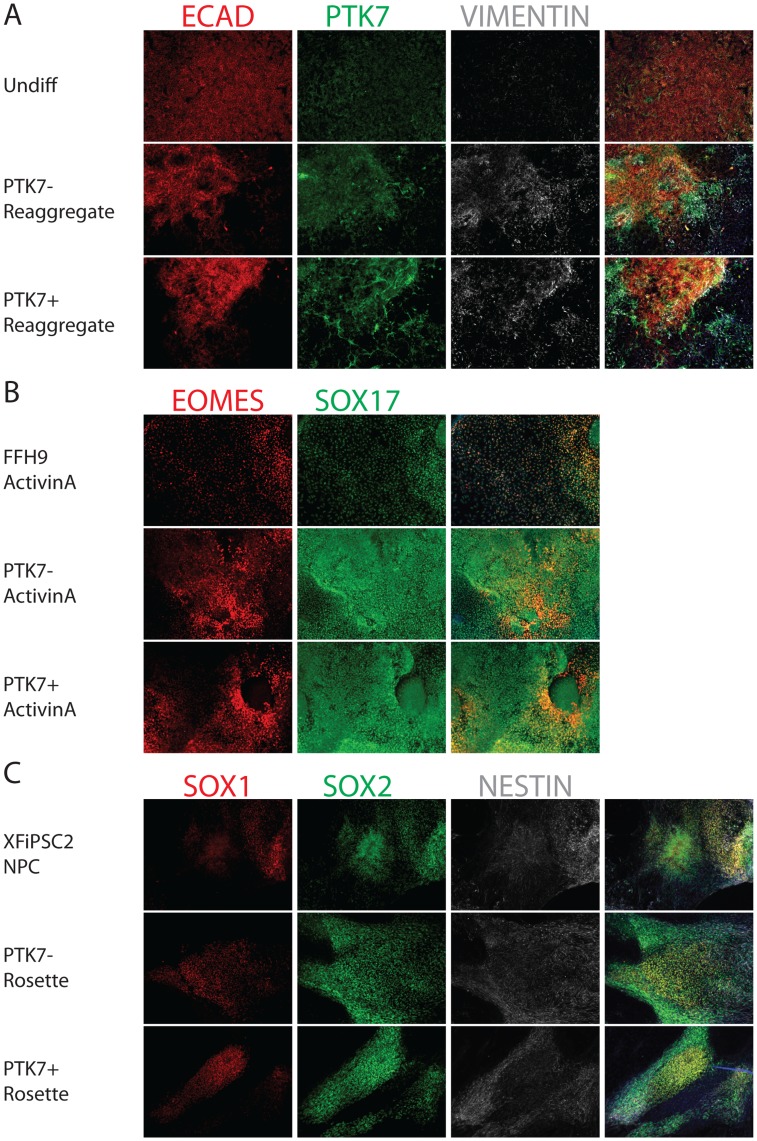
Figure 6. Cell Fate Determination on PTK7− and PTK7+ populations.
Feeder-free H9 (differentiated for 2 days) were sorted into PTK7+ and PTK7− populations. The two populations were re-aggregated as described in Materials and Methods, and replated onto Matrigel-coated coverslips. The PTK7− and PTK7+ re-aggregates were cultured in PSC media for 2 days, and then differentiated using established protocols. (A) Epithelial and mesenchymal marker analysis in PTK7− and PTK7+ re-aggregates 3 day post-sorting. PTK7+ cells that underwent developmental EMT possessed plasticity to revert back to an epithelial cell type. Undifferentiated H9 were plated on coverslips and fixed 4 days after passage. PTK7− and PTK7+ re-aggregates were plated on coverslips and fixed after 2 days in culture. Undifferentiated H9 (top row), PTK7− re-aggregate (mid row) and PTK7+ re-aggregate (bottom row) were stained for PTK7 (green), epithelial maker (E-CADHERIN, red) and mesenchymal marker (VIMENTIN, white). The 4th column shows the merged images from three fluorescent channels and DAPI. (B) PTK7+ and PTK7− re-aggregates exhibited comparable definitive endoderm formation. Feeder-free H9, PTK7− and PTK7+ re-aggregates were induced to form definitive endoderm with ActivinA treatment as described. Coverslips were fixed and immunostained with endodermal markers EOMES (red) and SOX17 (green). The 3rd column shows the merged images from two fluorescent channels and DAPI. (C) PTK7− and PTK7+ re-aggregates exhibited comparable neural differentiation. XFiPSC2, PTK7− and PTK7+ re-aggregates were induced to form neural rosettes as previously described. Coverslips were fixed and immunostained with neural markers SOX1 (red), SOX2 (green) and NESTIN (white). The 4th column shows the merged images from three fluorescent channels and DAPI. All images taken at 10X.