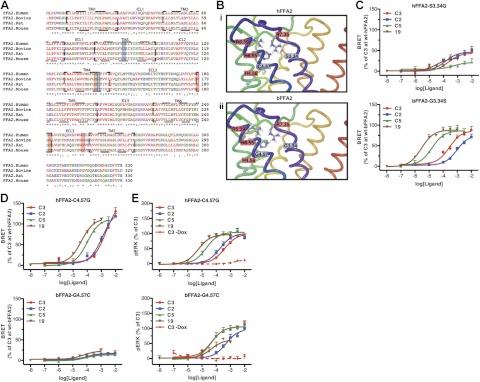
Figure 6.
Homology modeling and mutagenesis of bFFA2 indicate a key role for residue G4.57 in fatty acid chain length and compound selectivity. A, B) Species orthologs of FFA2 were aligned (A), and homology models of hFFA2 (Bi) and bFFA2 (Bii) were generated. Key amino acids known to play roles in the binding of SCFAs are boxed in red; those targeted for mutagenesis are boxed in blue. C, D) Residues at positions 3.34 (C) and 4.57 (D) were exchanged between the orthologs, and the ability of C2, C3, C5, and compound 19 to promote β-arrestin-2 recruitment to these variants was compared to wild type. E) hFFA2 and bFFA2 mutants with exchanged residues at 4.57 were assessed in an ERK 1/2 phosphorylation assay. To confirm responses were receptor mediated, C3 concentration-response studies were also carried out in the absence of doxycycline, to confirm it had no effect when receptor expression was not induced.