Figure 1.
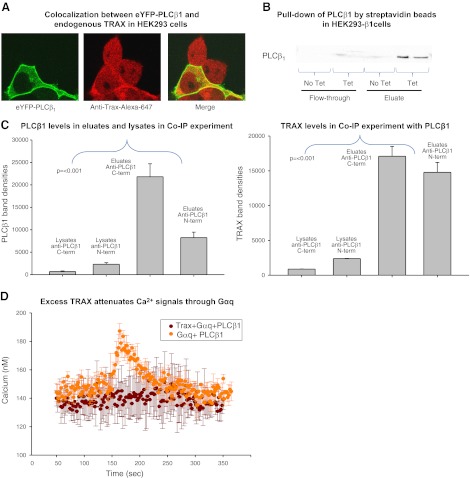
Evidence that PLCβ1 and TRAX associate in HEK293 cells. A) HEK293 cells expressing eYFP-PLCβ1 (left panel), and immunostained with anti-TRAX using an Alexa647 secondary antibody (middle panel). The figure is representative of 12 or more images and 4 independent studies. No bleed-through between the YFP and Alexa647 channels was detected. Degree of colocalization (yellow) of the two proteins is found in the cytosol and nucleus (right panel). B) Pulldown assay in which endogenous TRAX and PLCβ1, tagged on the N terminus with a strepavidin-binding peptide that was induced to express in HEK293-β1 cells, were copurified using beads coated with streptavidin (see Materials and Methods) and eluted in buffer containing biotin. C) Band intensity of PLCβ1 and TRAX pulled down by PLCβ1 through coimmunoprecipitation (Co-IP) in SKNSH cells. Two different PLCβ1 antibodies (Santa Cruz Biotechnology), one with an epitope at the C terminus and another at the N terminus, were used to pull down PLCβ1. Levels of PLCβ1 in the eluates were significantly higher than those in the lysates, indicating that PLC was being pulled down by the antibodies, with the C-terminal antibody having a higher affinity for PLCβ1. Higher levels of TRAX in the eluates indicate that TRAX was being concentrated by PLCβ1. We observed similar results from HEK cells (data not shown). D) Change in cellular Ca2+ with stimulation by 10 μM carbachol; cells overexpressing PLCβ1 and Gαq-eCFP (n=3) are shown in orange. Calcium levels in cells expressing Trax-eCFP, PLCβ1, and Gαq-eCFP (n=3) did not increase on stimulation and are shown in brown.