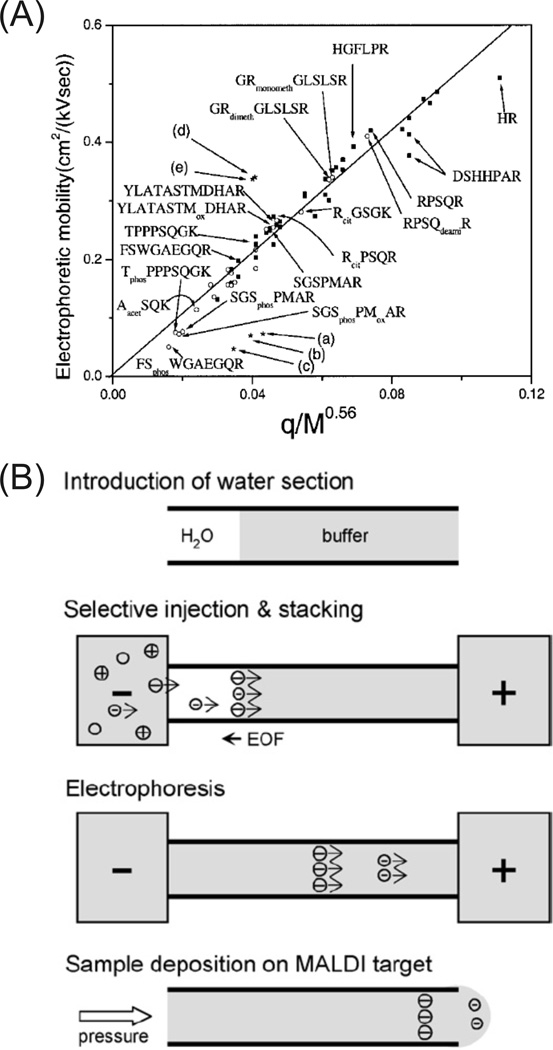
Figure 4.
(A) Correlation of electrophoretic mobility with q(charge)/Mr0.56 of peptides from a tryptic digest of myelin basic protein. Peptide mobilities with (■) and without (○) PTMs correlated well to the theoretical relationship. The phosphorylated peptides (phos) were grouped into a defined mobility window (< 0.1 cm2/kVsec). (B) An integrated procedure for phosphopeptide analysis. An injection of water is used to create a discontinuous electric field for selective injection and field amplified sample stacking of phosphopeptides. High mobility phosphopeptides (> EOF) stack into the water zone, are separated by electrophoresis, and deposited onto a MALDI target. (Modified from refs. (A) [68] and (B) [71] with permission. ©2003 Wiley-VCH and © 2006 Elsevier, respectively.)