ABSTRACT
BACKGROUND
Policy-makers have called for efforts to reduce overuse of cancer screening tests, including colorectal cancer screening (CRCS). Overuse of CRCS tests other than colonoscopy has not been well documented.
OBJECTIVE
To estimate levels and correlates of fecal occult blood test (FOBT) overuse in a national Veterans Health Administration (VHA) sample.
DESIGN
Observational
PARTICIPANTS
Participants included 1,844 CRCS-eligible patients who responded to a 2007 CRCS survey conducted in 24 VHA facilities and had one or more FOBTs between 2003 and 2009.
MAIN MEASURES
We combined survey data on race, education, and income with administrative data on region, age, gender, CRCS procedures, and outpatient visits to estimate overuse levels and variation. We coded FOBTs as overused if they were conducted <10 months after prior FOBT, <9.5 years after prior colonoscopy, or <4.5 years after prior barium enema. We used multinomial logistic regression models to examine variation in overuse by reason (sooner than recommended after prior FOBT; sooner than recommended after colonoscopy, barium enema, or a combination of procedures), adjusting for clustering of procedures within patients, and patients within facilities.
KEY RESULTS
Of 4,236 FOBTs received by participants, 885 (21 %) met overuse criteria, with 323 (8 %) sooner than recommended after FOBT, and 562 (13 %) sooner than recommended after other procedures. FOBT overuse varied across facilities (9–32 %, p < 0.0001) and region (12–23 %, p < .0012). FOBT overuse after prior FOBT declined between 2003 and 2009 (8 %–5 %, p = .0492), but overuse after other procedures increased (11–19 %, p = .0002). FOBT overuse of both types increased with number of outpatient visits (OR 1.15, p < 0.001), but did not vary by patient demographics. More than 11 % of overused FOBTs were followed by colonoscopy within 12 months.
CONCLUSIONS
Many FOBTs are performed sooner than recommended in the VHA. Variation in overuse by facility, region, and outpatient visits suggests addressing FOBT overuse will require system-level solutions.
KEY WORDS: colorectal neoplasms, mass screening, utilization, clinical practice variation, veterans
INTRODUCTION
As pressure to reduce health care expenditures mounts in this country, the value of preventive health care services such as cancer screening is being increasingly scrutinized. Some have claimed that the benefits of mammography have been overstated,1 others have documented excessive levels of cancer screening in populations unlikely to benefit from these services,2–8 and concern about overuse is beginning to receive attention in health policy circles. Indeed, in its initiative to transform health care delivery, the Veterans Health Administration (the nation’s largest integrated health care system) has called for a number of specific efforts to reduce inappropriate utilization of cancer screening, including reducing the number of fecal occult blood tests (FOBT) ordered for patients with evidence of a colonoscopy in the past 10 years.
The U.S. Preventive Services Task Force (USPSTF) currently recommends colorectal cancer screening (CRCS) for men and women age 50–75 using either FOBT annually, sigmoidoscopy every 5 years coupled with FOBT every 3 years, or colonoscopy every 10 years.9 Although recently removed from the list of CRCS modalities recommended by the USPSTF,9 double contrast barium enema every 5 years is still endorsed by some guideline-issuing bodies.10 Rates of adherence to CRSC guidelines have increased substantially in this country over the past decade,11 and in some settings12 have surpassed the Healthy People 2020 target of 70.5 %.13 In settings, such as the Veterans Health Administration (VHA), where CRCS rates exceed 80 %, attention is increasingly shifting away from addressing underutilization, toward documenting and ameliorating potential overuse of CRCS (e.g., screening individuals unlikely to benefit or screening more frequently than recommended by guidelines).14 Overuse of CRCS is important to address, because it can unnecessarily increase: (1) patient harm from overdiagnosis,15 including colonoscopy complications such as bowel perforation, gastrointestinal bleeding, serious cardiovascular events, and death;16,17 (2) demand for diagnostic colonoscopy (which remains in limited supply in many settings); and (3) health care costs.
Several studies have documented levels and variation in CRCS overuse attributable to screening individuals unlikely to benefit (such as those with limited life expectancy),2,7,18 and two studies have documented overuse of colonoscopy associated with shorter than recommended repeat screening19,20 and surveillance20 intervals (i.e., the timing of repeat colonoscopy following the removal of adenomatous or hyperplastic polyps.) To our knowledge, however, only two studies have examined CRCS overuse stemming from too frequent utilization of screening modalities other than colonoscopy.2,21 One of these studies was limited by the fact that it relied on physician self-reported rather than medical records-documented patterns of screening behavior,21 and the other was conducted in a single medical facility that may not generalize to other settings.2 Therefore, additional research is needed to describe the prevalence and determinants of overuse of CRCS for modes other than colonoscopy.
While colonoscopy has become the dominant screening modality in many U.S. health care settings, at least two integrated health care systems in the U.S. have achieved high CRCS rates based on programs emphasizing FOBT,12,14 and many countries outside of the U.S. still rely primarily on FOBT for CRCS.22–25 Furthermore, while admittedly of less concern from a patient safety or cost perspective than colonoscopy overuse, FOBT overuse is nevertheless important to document in settings that offer more than one CRSC modality, because it may affect demand for diagnostic colonoscopy, and reveal inefficiencies in the screening system that stem from lack of coordination across services that share responsibility for delivering and monitoring CRCS procedures.
The current study adds to the nascent literature on CRCS overuse, by examining levels and correlates of FOBT overuse in a nationally representative sample of patients receiving care from 24 VHA medical facilities that historically have relied primarily on FOBT for CRCS, but have increased use of screening colonoscopy over the past 5 years. Specifically, we: (1) estimate the extent of FOBT overuse related to screening frequency by reason (too soon after a prior FOBT versus too soon after other CRCS procedures), (2) determine whether overuse varies across facilities, regions, calendar year, or patient subgroups, and (3) document demand for colonoscopy associated with FOBT overuse.
METHODS
Participants
This study involved secondary analyses of data sources originally assembled for the purpose of assessing patient variation in the underuse of CRCS. Participants were CRCS-eligible patients recruited to participate in a survey of CRCS behavior.26 The survey cohort was drawn from the population of male and female patients age 50 to 75 years who had one or more primary care visits between January 2005 and December 2006 at one of 124 VHA medical facilities. Employees, deceased individuals, and patients either enrolled in VHA adult day care or nursing home facilities, or with documentation of colorectal cancer (CRC), dementia, or Alzheimer’s disease in VHA medical records were excluded.
Sampling
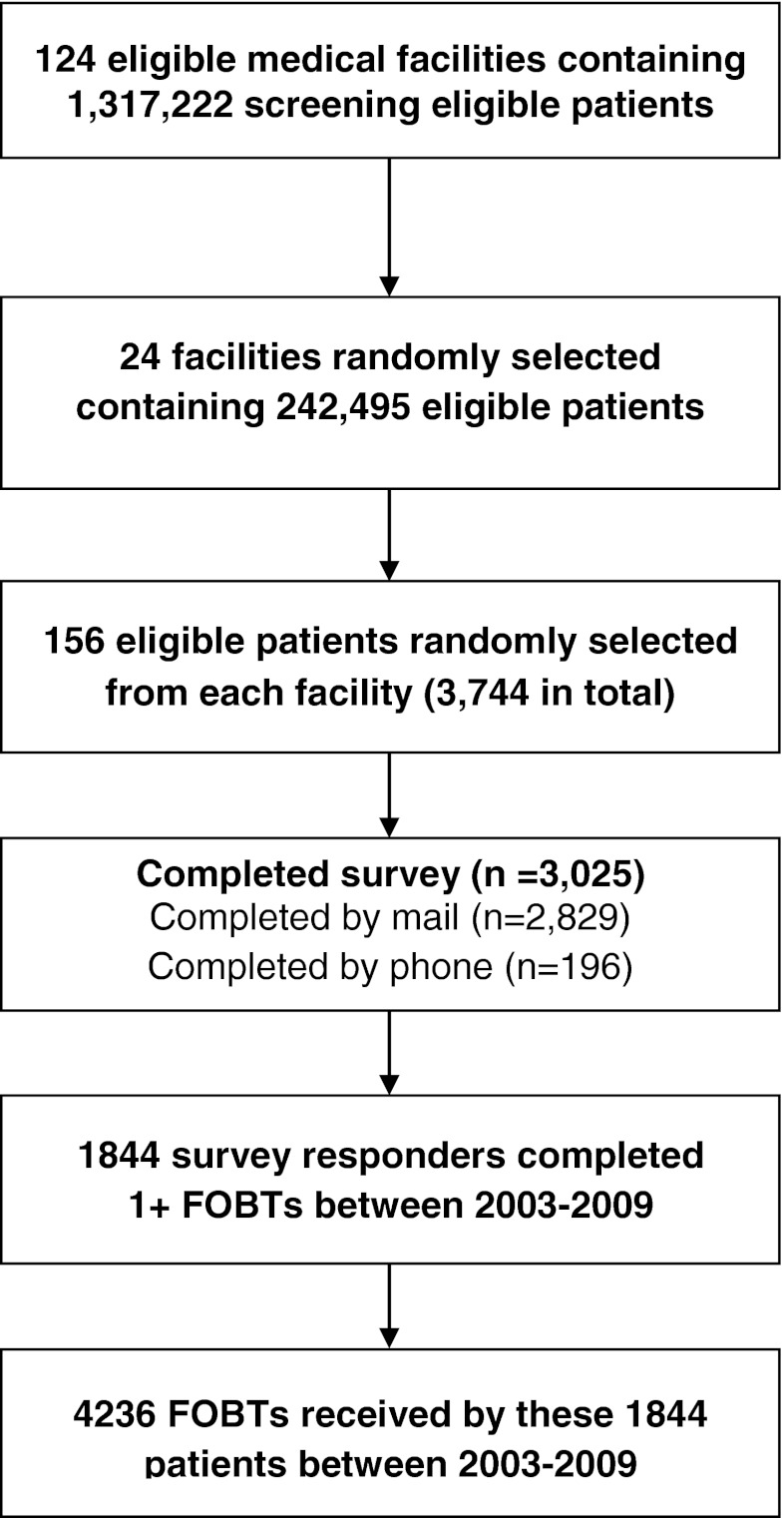
A two-staged hierarchical sampling strategy was used to select the survey sample (Fig. 1). In the first stage, the 124 eligible VHA facilities were grouped into 12 strata (based on size of the eligible patient population and proportion of African American patients), and two facilities were randomly selected from each stratum (yielding 24 facilities). In the second stage, a simple random sample of 156 eligible patients was selected from each of the 24 sampled facilities, generating a sample of 3,744 patients.
Figure 1.
Subject flow diagram.
Data Collection
These 3,744 patients were recruited to a survey on CRCS behavior between February 2007 and May 2007. Administration of the survey involved an initial mailing including a cover letter, a 15-page questionnaire (available at http://www.hsrd.minneapolis.med.va.gov/PDF/SCREEN_NationalSurvey.pdf), and $2 cash incentive. A reminder postcard was mailed 1 week after the first survey mailing. A second survey mailing (with no incentive) was mailed to those who did not return a questionnaire within 3–4 weeks of the first mailing. Phone administration was attempted for those who did not return a questionnaire within 3 weeks of the second survey mailing. A total of 3,025 (81 %) patients completed the survey.
The 1,844 survey respondents who received one or more FOBTs at a VHA facility between 2003 and 2009 were included in the analysis. The 4,236 FOBTs received by these participants were evaluated for overuse. VHA medical records information on patient demographics, diagnoses, and CRCS procedures received at the VHA were available for all participants. These data and facility-level information on region and organizational complexity were linked to patient survey data to examine variation in overuse. Physician variation was not examined because the number of screening tests per physician was too small to derive reliable estimates.
Dependent Measures
The primary dependent measure was FOBT overuse. We coded FOBTs as overused if they were conducted <10 months after a prior FOBT, <9.5 years after a prior colonoscopy, or <4.5 years after a prior barium enema. We used these intervals, rather than the screening intervals recommended by the USPSTF (12 months, 10 years, and 5 years, respectively), to account for the fact that preventing a lapse in screening adherence may require ordering a test a few months before the patient would become overdue for screening. FOBTs conducted within 30 days of a prior FOBT were excluded. We did not consider sigmoidoscopies in our definition of overuse because until November 2008, annual FOBT coupled with sigmoidoscopy every 5 years was a USPSTF-endorsed CRCS strategy.27 Therefore, between 2003 and 2008, an FOBT following a sigmoidoscopy could only be considered unnecessary if there was also an FOBT <10 months prior to the index FOBT (a criterion that is redundant with the above overuse criterion for an FOBT following a prior FOBT). Beginning in 2009, if one was following the current USPSTF guidelines9 and applying similar criteria as above, an FOBT following a sigmoidoscopy could be considered overused if the sigmoidoscopy was less than 4.5 years prior and there was also an FOBT less than two years and 10 months prior. However, incorporating this information into our definition of FOBT overuse would have changed the status of only three FOBTs conducted in 2009 from “not overused” to “overused”. Finally, we did not attempt to eliminate FOBTs conducted for diagnostic reasons, since prior research in the VHA suggests FOBTs are rarely used for non-screening indications,28 and excluding non-screening FOBTs from overuse estimates does not alter estimates.29
We used multinomial logistic regression models to examine facility and patient variation in FOBT overuse by reason (sooner than recommended after prior FOBT; sooner than recommended after colonoscopy, barium enema, or a combination of procedures). Estimates were adjusted for the clustering of procedures within patients and facilities. To document demand for colonoscopy associated with FOBT overuse, we also estimated the number and proportion of FOBTs flagged for overuse that were followed by a colonoscopy within 12 months.
Covariates
Patient characteristics obtained from VHA medical records included: age (50–64 versus 65–75 years), gender, and utilization (number of outpatient visits in the year of and year prior to the FOBT). Patient characteristics obtained from the survey included race (white versus non–white), education (≤ high school, versus > high school), and income (≤$20,000, $20–40,000, >$40,000). Region (southwest versus Midwest, northeast, northwest, and south) of the facility where the FOBT was conducted was included as a covariate. Whether the FOBT was completed by a patient who receives some care outside of the VHA, and facility complexity (a summary measure based on facility size, intensive care capacity, levels of patient complexity, teaching and research activity, and number of specialists)30,31 were included as controls in our analyses since both have been previously found to be correlated with CRCS rates26,32 and may vary by region.
Analysis
We calculated FOBT overuse point estimates and 95 % confidence intervals overall and by year, facility, and reason (sooner than recommended after prior FOBT, colonoscopy, barium enema, or combination of procedures). Given the stratified two-stage sampling design, we used design-weighted logistic regression models accounting for the stratification and clustering by facility to derive these overuse estimates and to examine the association of overuse with patient and facility characteristics. As described elsewhere,33 the sample design weights were adjusted to account for survey non-response, using reweighting within propensity of response strata. Standard errors for the estimates were obtained from bootstrapping the within facility samples. Adjusted estimates of the odds of FOBT overuse by reason were derived from similar design weighted multinomial logistic regression models, controlling for facility complexity and patient use of non-VHA health care. The unit of analysis for these regressions was the FOBT test, and estimates adjusted for the clustering of procedures within patients, and patients within facilities. Among survey respondents, patient race, education, income, and receipt of non-VHA health care derived from the survey had 1 %, 4 %, 9 %, and 6 % missing responses, respectively. To avoid biases and power reductions that could result from dropping cases with missing values on any of these characteristics from the analyses, we used multiple imputation procedures34,35 to replace each missing value with ten plausible values (i.e., creating ten imputed data sets) and combined results from analyzing each imputed data set using standard methods for multiple imputation. The source of variation stemming from the uncertainty in imputing for missing values is reflected in our estimated standard errors and accompanying confidence intervals.
The study was approved by the Minneapolis VHA Medical Center Institutional Review Board.
RESULTS
The majority of patients in the sample were white (78 %) males (96 %) between the ages of 50–64 (61 %), with incomes ≤$40,000 (73 %), and many (49 %) had more than a high school education (Table 1). The average number of outpatient visits in the year of and the year prior to sampled FOBTs was 3.7 per participant, and the majority of participants received their FOBTs from facilities in the northeast (34 %) and south (32 %).
Table 1.
Patient Characteristics (n = 1,844)
| Characteristic | N | % |
|---|---|---|
| Age | ||
| 50–64 | 1,126 | 61.1 |
| 65 and older | 718 | 38.9 |
| Race | ||
| American Indian/Alaskan Native | 76 | 4.1 |
| Asian | 9 | 0.5 |
| African American | 235 | 12.7 |
| Native Hawaiian/Pacific Islander | 3 | 0.2 |
| White | 1,432 | 77.7 |
| Other | 67 | 3.6 |
| Unknown | 22 | 1.2 |
| Ethnicity | ||
| Hispanic | 50 | 2.7 |
| Gender | ||
| Female | 74 | 4.0 |
| Male | 1,770 | 96.0 |
| Education | ||
| ≤High school | 866 | 47.0 |
| >High school | 907 | 49.2 |
| Unknown | 71 | 3.9 |
| Annual household income | ||
| ≤$20,000 | 742 | 40.2 |
| $20,001–$40,000 | 608 | 33.0 |
| ≥$40,001 | 347 | 18.8 |
| Unknown | 147 | 8.0 |
| Utilization | Mean = 3.7 (range 0–38) | |
| Region | ||
| Mid West | 203 | 11.0 |
| North East | 624 | 33.8 |
| North West | 242 | 13.1 |
| South | 585 | 31.7 |
| South West | 190 | 10.3 |
Of the 4,236 FOBTs received by our cohort, 885 (21 %) met our criteria for overuse; 323 (8 %) because they were conducted sooner than recommended after FOBT, and 562 (13 %) because they were conducted sooner than recommended after other procedures (colonoscopy, barium enema, or combination) (Table 2). Among FOBTs conducted too soon following prior FOBTs, the average time since the prior FOBT was 6.7 months (or 204 days). Among FOBTs conducted too soon following other procedures, the average interval between procedures was 36 months (or 3 years); 37 months when limited to prior colonoscopy procedures.
Table 2.
Number and Percent of FOBTs by Overuse Status and Reason
| Reason | N (%) |
|---|---|
| FOBT meeting overuse criteria | 885 (20.89) |
| Too soon after prior FOBT | 323 (7.63) |
| Too soon after prior Barium Enema | 76 (1.79) |
| Too soon after prior FOBT and Barium Enema | 6 (0.14) |
| Too soon after prior Colonoscopy | 394 (9.30) |
| Too soon after prior FOBT and Colonoscopy | 45 (1.06) |
| Too soon after prior Colonoscopy and Barium Enema | 32 (0.76) |
| Too soon after prior FOBT, Barium Enema, and Colonoscopy | 9 (0.21) |
| FOBT not meeting overuse criteria | 3,351 (79.11) |
| TOTAL FOBT | 4,236 (100.00) |
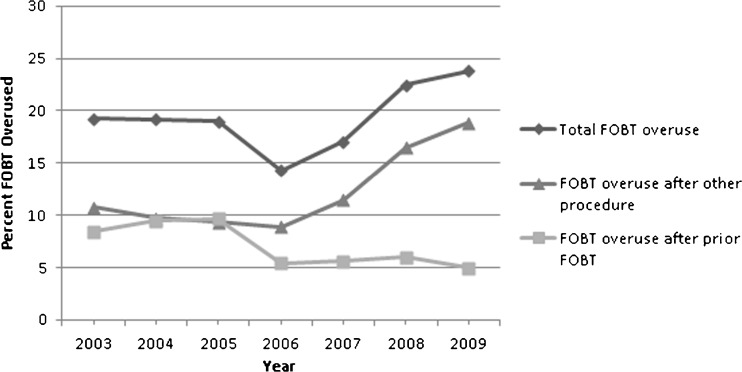
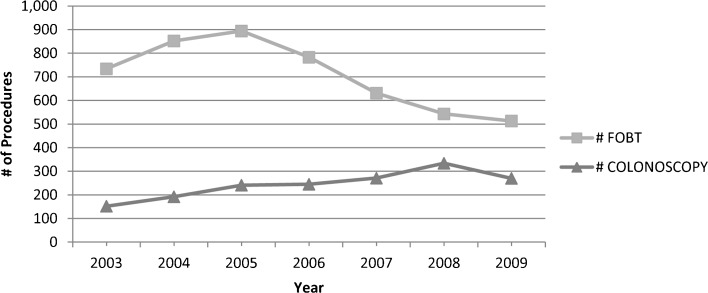
Total FOBT overuse varied significantly across facilities (9–32 %, p < 0.0001), and region (12–23 %, p = 0.0012), and increased over time (from 19 % in 2003 to 24 % in 2009), but not significantly so (p = 0.1001). As shown in Figure 2, FOBT overuse after prior FOBT declined significantly between 2003 and 2009 (from 8 % to 5 %, p = 0.0492), while FOBT overuse after other procedures increased over this same time period (from 11 to 19 %, p = 0.0002). Therefore, the modest increase in total FOBT overuse over time is due entirely to significant increases in overuse following prior colonoscopies and other procedures. Over this same time period, the number of colonoscopies received by study participants increased dramatically, while the number of FOBTs declined (Fig. 3).
Figure 2.
Percent of FOBT overused, by reason and year.
Figure 3.
Number of FOBT and colonoscopy procedures by year.
The adjusted odds of FOBT overuse did not vary significantly by patient demographic characteristics, but did vary significantly by number of outpatient visits and region (Table 3). The odds of overuse after prior FOBT increased with number of outpatient visits (OR 1.16, p < 0.0001), and were significantly lower in the Midwest compared to the Southwest (OR 0.29, p = 0.01). The odds of overuse after other procedures also increased with number of outpatient visits (OR 1.16, p < 0.0001), and were significantly lower in the Midwest (OR 0.41 p = 0.008) and Northwest (0.42 p = 0.009) compared to the Southwest.
Table 3.
Odds of FOBT Overuse, by Reason and Characteristics of Patients and Facilities (Significant Odds Ratios in Bold)
| Characteristic | Too soon after FOBT | Too soon after other procedure | ||
|---|---|---|---|---|
| Unadjusted odds ratio 95 % confidence interval) | Adjusted odds ratio (95 % confidence interval)* | Unadjusted odds ratio (95 % confidence interval) | Adjusted odds ratio (95 % confidence interval)* | |
| Age 65 and older | 1.06 (0.78, 1.43) | 1.02 (0.75, 1.40) | 1.03 (0.75, 1.41) | 1.09 (0.81, 1.48) |
| Race White | 1.06 (0.73, 1.54) | 1.09 (0.72, 1.65) | 1.06 (0.71, 1.57) | 1.31 (0.88, 1.95) |
| Gender Male | 1.11 (0.52, 2.40) | 1.16 (0.58, 2.31) | 0.55 (0.28, 1.10) | 0.57 (0.26, 1.25) |
| Education | ||||
| ≤High school | 1.08 (0.78, 1.51) | 0.89 (0.63, 1.26) | 1.26 (0.93, 1.71) | 1.24 (0.92, 1.68) |
| >High school | – | – | – | – |
| Annual household income | ||||
| $0–$20,000 | 1.15 (0.72, 1.83) | 1.13 (0.71, 1.81) | 1.36 (0.77, 2.39) | 1.12 (0.56, 2.23) |
| $20,000–$40,000 | 1.27 (0.75, 2.15) | 1.24 (0.78, 1.98) | 1.34 (0.81, 2.23) | 1.18 (0.65, 2.13) |
| >$40,000 | – | – | – | – |
| Utilization | 1.14 (1.10, 1.19) | 1.16 (1.11, 1.21) | 1.15 (1.11, 1.19) | 1.16 (1.10, 1.21) |
| Region | ||||
| Midwest | 0.39 (0.17, 0.94) | 0.29 (0.11, 0.77) | 0.52 (0.31, 0.88) | 0.41 (0.21, 0.77) |
| Northeast | 1.25 (0.67, 2.33) | 0.96 (0.49, 1.84) | 0.77 (0.49, 1.20) | 0.70 (0.42, 1.16) |
| Northwest | 0.59 (0.28, 1.25) | 0.45 (0.20, 1.02) | 0.41 (0.24, 0.72) | 0.42 (0.23, 0.78) |
| Southern States | 1.06 (0.56, 2.00) | 0.87 (0.45, 1.70) | 0.76 (0.50, 1.14) | 0.74 (0.46, 1.19) |
| Southwest | – | – | – | – |
*Adjusting for facility complexity, whether patient receives health care outside of the VHA, and all characteristics in column 1
The analyses to explore potential increased demand for colonoscopy associated with FOBT overuse revealed that 52 (5.58 %) of FOBTs flagged for overuse were followed by a colonoscopy within 3 months, 96 (10.85 %) within 10 months, and 102 (11.53 %) within 12 months.
DISCUSSION
We found that 21 % of FOBTs performed in the VHA are completed sooner than is considered necessary by guidelines. Although the odds of FOBT overuse did not vary by patient demographics, they did increase by 16 % with each additional outpatient visit. This pattern is consistent with prior studies documenting a positive association between health care utilization and CRCS.7,36–40 Given the strong association between chronic disease burden and health care utilization,41 the correlation between outpatient visits and FOBT overuse found in the current study may explain the pattern documented by others, whereby patients with significant comorbidities who are unlikely to benefit from CRCS nevertheless receive screening procedures.2,7,18
The FOBT overuse rates observed in this study are higher than those reported in the one prior published study examining FOBT overuse in the VHA.2 In their study conducted in a single VHA medical facility, Fisher and colleagues2 found that 5 % of patients receiving an FOBT had a colonoscopy less than 5 years prior. The differences in FOBT overuse observed in our studies may reflect differences in overuse definitions (5 year versus 9.5 year window for classifying FOBTs following a prior colonoscopy as overused), samples examined (single versus nationally representative sample of facilities), or temporal changes in CRCS patterns in the VHA (as the study by Fisher and colleagues was conducted prior to the increases in colonoscopy use within the VHA). Indeed, our findings suggest the sources and implications of FOBT overuse are closely tied to colonoscopy utilization patterns.
An important finding from this study is that the increases in FOBT overuse between 2003 and 2009 in the VHA setting were entirely attributable to increases in FOBT conducted too soon after a prior colonoscopy, as the occurrence of FOBT too soon after a prior FOBT declined over this time period. The frequency with which FOBT tests are conducted too soon following prior colonoscopies may be tied to characteristics of the computerized clinical reminders widely used within the VHA to promote adherence to CRCS guidelines.42 Originally developed when FOBT was the dominant screening modality in the VHA, and designed to prompt providers at each encounter until they are resolved by ordering a CRCS test, these reminders may not have been as effectively adapted in all settings to allow modified prompting schedules for patients receiving colonoscopies. Indeed, variation in whether and how clinical reminders have been modified to adapt to the increasing use of colonoscopy may be one of the system factors that explains the significant variation across facilities and regions in FOBT overuse that we observed in this study. Another possible explanation why FOBTs are conducted sooner than recommended following prior colonoscopies is that providers disagree with the 10 year interval recommended by guidelines. However, national studies suggest high levels of primary care provider agreement with the annual FOBT screening interval,21,43 and a recent study found that 96 % of VHA gastroenterologists performed colonoscopy 10 years after a normal colonoscopy, suggesting high agreement with the recommended screening colonoscopy interval.44
While FOBT (typically estimated at <$60 per procedure)45 is low cost and poses little risk to patients, overuse of this procedure may generate unwarranted demand for high cost, invasive follow-up colonoscopies (typically estimated at >$1,500 per procedure),45 which remain in limited supply nationally,46 and can lead to serious patient harms (including bowel perforation, bleeding, and adverse cardiovascular events).16,17 Indeed, we found that more than 11 % of FOBTs flagged for overuse in our cohort were followed by a colonoscopy within 12 months. If appropriate screening intervals had been followed, some of these procedures would have been postponed and some would not have occurred at all.
Our findings should be qualified by the following limitations. First, because our data did not include information on colonoscopies received outside of the VHA system by patients in our cohort, and prior studies suggest that a sizable fraction of colonoscopies received by veterans are performed outside of the VHA,47 our results may underestimate actual levels of FOBT overuse following colonoscopy. Indeed, if the rate of outside colonoscopy usage in our national sample is similar to that reported in a prior study conducted on the Minneapolis VHA,47 our overuse estimates would increase by approximately five percentage points. Second, because our data did not include information on colonoscopy results, we were unable to examine CRCS overuse related to repeat colonoscopy; a problem well documented by several prior studies.18,20,21 Third, we were not able to examine physician variation in overuse patterns. Because strategies to reduce overuse will differ depending on the extent to which variation in rates is associated with providers versus facilities, future studies should quantify the relative contribution of these two sources of variation. Finally, inappropriate use of FOBT among individuals with a family history of CRC (for whom colonoscopy is the recommended test), and overuse of CRCS due to screening individuals unlikely to benefit, such as individuals with limited life expectancy, were not examined in this study.
In conclusion, many FOBTs conducted in the VHA are performed sooner than recommended. This overuse generates unnecessary demand for colonoscopy and varies by facility, region, and outpatient utilization. This variation suggests that addressing FOBT overuse will likely require system-level solutions.
Acknowledgments
Dr. Partin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The study was supported by VA Health Services Research & Development grants #PPO 09-292 (Partin) and #CDA 08-024 (Powell). The funding organization had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Welch HG, Frankel BA. Likelihood that a woman with screen-detected breast cancer has had her “life saved” by that screening. Arch Intern Med. 2011;171(22):2043–2046. doi: 10.1001/archinternmed.2011.476. [DOI] [PubMed] [Google Scholar]
- 2.Fisher DA, Judd L, Sanford NS. Inappropriate colorectal cancer screening: findings and implications. Am J Gastroenterol. 2005;100(11):2526–2530. doi: 10.1111/j.1572-0241.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 3.Meissner HI, Tiro JA, Haggstrom D, Lu-Yao G, Breen N. Does patient health and hysterectomy status influence cervical cancer screening in older women? J Gen Intern Med. 2008;23(11):1822–1828. doi: 10.1007/s11606-008-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sirovich BE, Welch HG. Cervical cancer screening among women without a cervix. JAMA. 2004;291(24):2990–2993. doi: 10.1001/jama.291.24.2990. [DOI] [PubMed] [Google Scholar]
- 5.Walter LC, Davidowitz NP, Heineken PA, Covinsky KE. Pitfalls of converting practice guidelines into quality measures: lessons learned from a VA performance measure. JAMA. 2004;291(20):2466–2470. doi: 10.1001/jama.291.20.2466. [DOI] [PubMed] [Google Scholar]
- 6.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296(19):2336–2342. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 7.Walter LC, Lindquist K, Nugent S, Schult T, Lee SJ, Casadei MA, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150(7):465–473. doi: 10.7326/0003-4819-150-7-200904070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sima CS, Panageas KS, Schrag D. Cancer screening among patients with advanced cancer. JAMA. 2010;304(14):1584–1591. doi: 10.1001/jama.2010.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Preventive Services Task Force. Screening for colorectal cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2008;149(9):627–37. [DOI] [PubMed]
- 10.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Richardson LC, Tai E, Rim SH, Joseph D, Plescia M. Vital signs: colorectal cancer screening, incidence, and mortality–United States, 2002–2010. MMWR Morb Mortal Wkly Rep. 2011;60(26):884–889. [PubMed] [Google Scholar]
- 12.National Institutes of Health. NIH state-of-the-science conference: enhancing use and quality of colorectal cancer screening - program and abstracts. Bethesda, MD; February 2–4, 2010. Available at: http://consensus.nih.gov/2010/images/colorectal/colorectal_abstracts.pdf#pagemode=bookmarks&page=1. Accessed June 21, 2012.
- 13.U.S.Department of Health and Human Services. Healthy People 2020. Office of Disease and Health Promotion. Washington, DC. Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf Accessed June 21, 2012.
- 14.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, et al. NIH state-of-the-science conference statement on enhancing use and quality of colorectal cancer screening. NIH. 2010;27(1):1–31. [PubMed] [Google Scholar]
- 15.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 16.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–857. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 18.Kahi CJ, van Ryn M, Juliar B, Stuart JS, Imperiale TF. Provider recommendations for colorectal cancer screening in elderly veterans. J Gen Intern Med. 2009;24(12):1263–1268. doi: 10.1007/s11606-009-1110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin JS, Singh A, Reddy N, Riall TS, Kuo YF. Overuse of screening colonoscopy in the medicare population. Arch Intern Med. 2011;171(15):1335–1343. doi: 10.1001/archinternmed.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krist AH, Jones RM, Woolf SH, Woessner SE, Merenstein D, Kerns JW, et al. Timing of repeat colonoscopy: disparity between guidelines and endoscopists’ recommendation. Am J Prev Med. 2007;33(6):471–478. doi: 10.1016/j.amepre.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 21.Yabroff KR, Klabunde CN, Yuan G, McNeel TS, Brown ML, Casciotti D, et al. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med. 2010;26(2):177–184. doi: 10.1007/s11606-010-1516-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoff G, Dominitz JA. Contrasting US and European approaches to colorectal cancer screening: which is best? Gut. 2010;59(3):407–414. doi: 10.1136/gut.2009.192948. [DOI] [PubMed] [Google Scholar]
- 23.The Multicentre Australian Colorectal-neoplasia Screening Group (MACS) A comparison of colorectal neoplasia screening tests: a multicentre community-based study of the impact of consumer choice. Med J Aust. 2006;184(11):546–550. doi: 10.5694/j.1326-5377.2006.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 24.Kanavos P, Schurer W. The dynamics of colorectal cancer management in 17 countries. Eur J Health Econ. 2010;10(Suppl 1):S115–S129. doi: 10.1007/s10198-009-0201-2. [DOI] [PubMed] [Google Scholar]
- 25.Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122(6):1357–1367. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- 26.Partin MR, Noorbaloochi S, Grill J, Burgess DJ, van Ryn M, Fisher DA, et al. The interrelationships between and contributions of background, cognitive, and environmental factors to colorectal cancer screening adherence. Cancer Causes Control. 2010;21(9):1357–1368. doi: 10.1007/s10552-010-9563-0. [DOI] [PubMed] [Google Scholar]
- 27.Preventive Services US. Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137(2):129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 28.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 29.Fisher DA, Galanko J, Dudley TK, Shaheen NJ. Impact of comorbidity on colorectal cancer screening in the veterans healthcare system. Clin Gastroenterol Hepatol. 2007;5(8):991–996. doi: 10.1016/j.cgh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Stefos T, LaVellee N, Holden F. Fairness in prospective payment: a clustering approach. Health Serv Res. 1992;27(2):239–261. [PMC free article] [PubMed] [Google Scholar]
- 31.Szabo CR. 2005 Facility Complexity Model. Veterans Health Administration. Washington: NLB Human Resources Committee; 2005. [Google Scholar]
- 32.Yano EM, Soban LM, Parkerton PH, Etzioni DA. Primary care practice organization influences colorectal cancer screening performance. Health Serv Res. 2007;42(3 Pt 1):1130–1149. doi: 10.1111/j.1475-6773.2006.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess DJ, van Ryn M, Grill J, Noorbaloochi S, Griffin JM, Ricards J, et al. Presence and correlates of racial disparities in adherence to colorectal cancer screening guidelines. J Gen Intern Med. 2011;26(3):251–258. doi: 10.1007/s11606-010-1575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New York: John Wiley; 2003. [Google Scholar]
- 35.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 36.Hahn DL. The delivery of clinical preventive services: acute care intervention. J Fam Pract. 1999;48(10):785–789. [PubMed] [Google Scholar]
- 37.Ioannou GN, Chapko MK, Dominitz JA. Predictors of colorectal cancer screening participation in the United States. Am J Gastroenterol. 2003;98(9):2082–2091. doi: 10.1111/j.1572-0241.2003.07574.x. [DOI] [PubMed] [Google Scholar]
- 38.Kodl MM, Powell AA, Noorbaloochi S, Grill JP, Bangerter AK, Partin MR. Mental health, frequency of healthcare visits, and colorectal cancer screening. Med Care. 2010;48(10):934–939. doi: 10.1097/MLR.0b013e3181e57901. [DOI] [PubMed] [Google Scholar]
- 39.Ruffin MT, Gorenflo DW, Woodman B. Predictors of screening for breast, cervical, colorectal, and prostatic cancer among community-based primary care practices. J Am Board Fam Pract. 2000;13(1):1–10. doi: 10.3122/jabfm.13.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman RK, Nowalk MP, Tabbarah M, Grufferman S. Predictors of colorectal cancer screening in diverse primary care practices. BMC Health Serv Res. 2006;6:116. doi: 10.1186/1472-6963-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Chao HH, Schwartz AR, Hersh J, Hunnibell L, Jackson GL, Provenzale DT, et al. Improving colorectal cancer screening and care in the Veterans Affairs Healthcare system. Clin Colorectal Cancer. 2009;8(1):22–28. doi: 10.3816/CCC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 43.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006–2007. Am J Prev Med. 2009;37(1):8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah TU, McNeil R, Wu R, Fisher DA. Understanding gastroenterologist adherence to polyp surveillance guidelines. Am J Gastroenterol. In press. [DOI] [PMC free article] [PubMed]
- 45.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33(1):88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 47.Partin MR, Grill J, Noorbaloochi S, Powell AA, Burgess DJ, Vernon SW, et al. Validation of self-reported colorectal cancer screening behavior from a mixed-mode survey of veterans. Cancer Epidemiol Biomarkers Prev. 2008;17(4):768–776. doi: 10.1158/1055-9965.EPI-07-0759. [DOI] [PubMed] [Google Scholar]