Fig. 3.
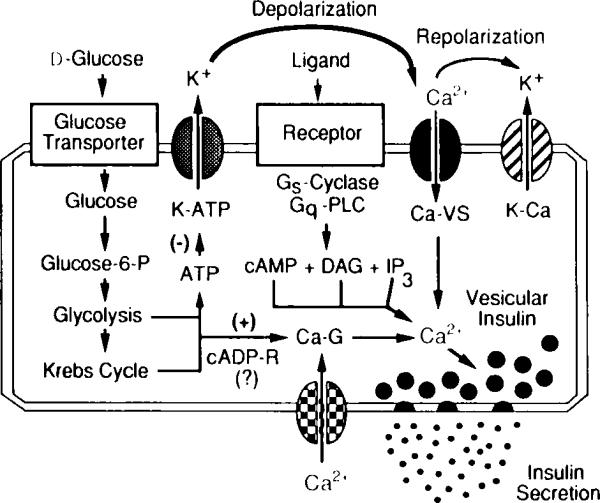
Signaling systems that regulate intracellular Ca2+ and insulin secretion from pancreatic β cells. Illustrated are essential features of the glucose signaling system (left); the hormonally regulated 3′,5′-cyclic adenosine monophosphate (cAMP), inositol trisphosphate IP3), and diacylglycerol (DAG) signaling systems (middle); and the cyclic ADP–ribose signaling system (bottom) that act in concert to trigger insulin secretion. The initial uptake of glucose is facilitated by the type-2 glucose transporter, whereas the conversion of glucose to glucose 6-phosphate is catalyzed by glucokinase. Stimulation of aerobic glycolysis generates multiple signals, one of which is an increased ratio of intracellular ATP relative to ADP. Binding of ATP to ATP-sensitive potassium channels (K–ATP) induces closure of the channels and membrane depolarization, which is necessary for the opening of L-type voltage-sensitive Ca2+ channels (Ca–VS). The glucose-induced rise in Ca2+ also results from the action of glucose-derived metabolites to open G-type voltage-independent Ca2+ channels (Ca–G). In this figure, one such metabolite is proposed to be cyclic ADP–ribose (cADP-R). Entry of Ca2+ across the plasma membrane triggers vesicular insulin secretion by Ca2+-dependent exocytosis. Repolarization results from the action of cytosolic Ca2+ to activate Ca2+-dependent potassium channels (K–Ca) and to inhibit voltage-sensitive Ca2+ channels. Also illustrated are hormonally regulated second messenger signaling systems that act through GTP-binding protein (Gs,Gq)-coupled receptors to stimulate production of cAMP (a messenger generated by adenylyl cyclase) and IP3 or DAG (messengers generated by phospholipase C, PLC). These messengers increase cytosolic Ca2+ by facilitating the opening of Ca–VS and by mobilizing Ca2+ from intracellular Ca2+ stores.
