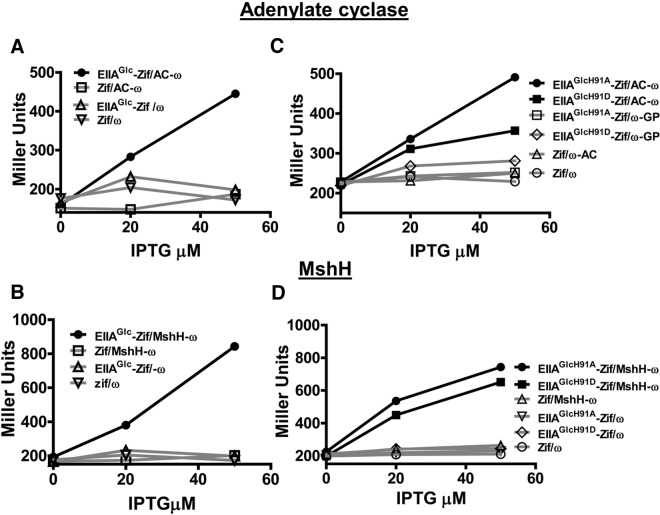
FIG 2.
Bacterial two-hybrid assays confirm the interaction of adenylate cyclase and MshH with native EIIAGlc and EIIAGlc point mutants mimicking the phosphorylated and unphosphorylated states. (A and B) Interaction of EIIAGlc fused to the N terminus of the DNA-binding protein Zif (EIIAGlc-Zif) with adenylate cyclase (AC-ω) (A) or MshH (MshH-ω) (B) fused to the ω subunit of RNA polymerase in an E. coli strain encoding the lacZ gene preceded by a Zif binding site. (C and D) A similar experiment was performed to assess the interactions of adenylate cyclase (AC-ω) (C) or MshH (MshH-ω) (D) with EIIAGlc point mutants in which an alanine (EIIAGlcH91A-Zif) or an aspartate (EIIAGlcH91D-Zif) was substituted for histidine 91 to mimic the unphosphorylated and phosphorylated states of EIIAGlc, respectively. Controls, which are traced in gray for each experiment, include (i) a vector encoding Zif alone (Zif) combined with a vector encoding the protein of interest fused to ω, (ii) a vector encoding native EIIAGlc or point mutants fused to Zif combined with a vector encoding ω alone (ω), and (iii) vectors encoding Zif and ω alone. Two experimental replicates were included in each trial, and several trials were performed. A representative trial is shown here.