Abstract
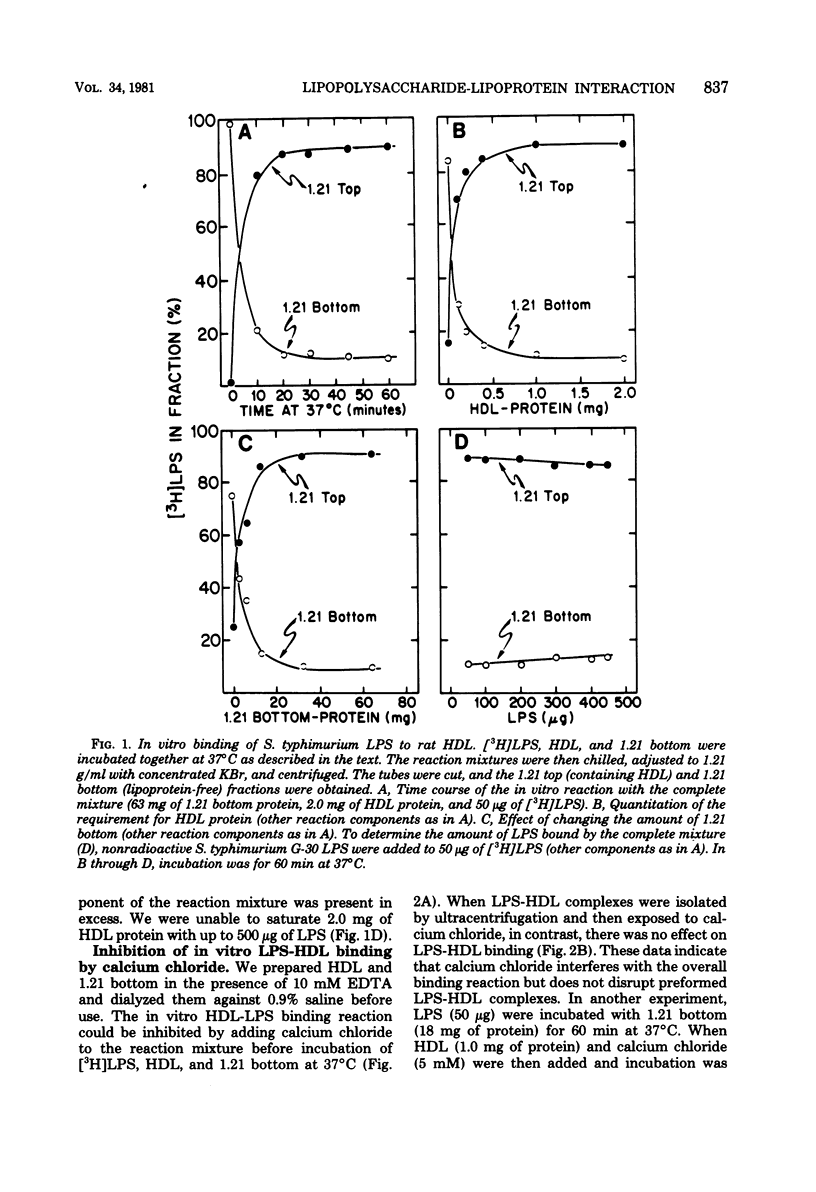
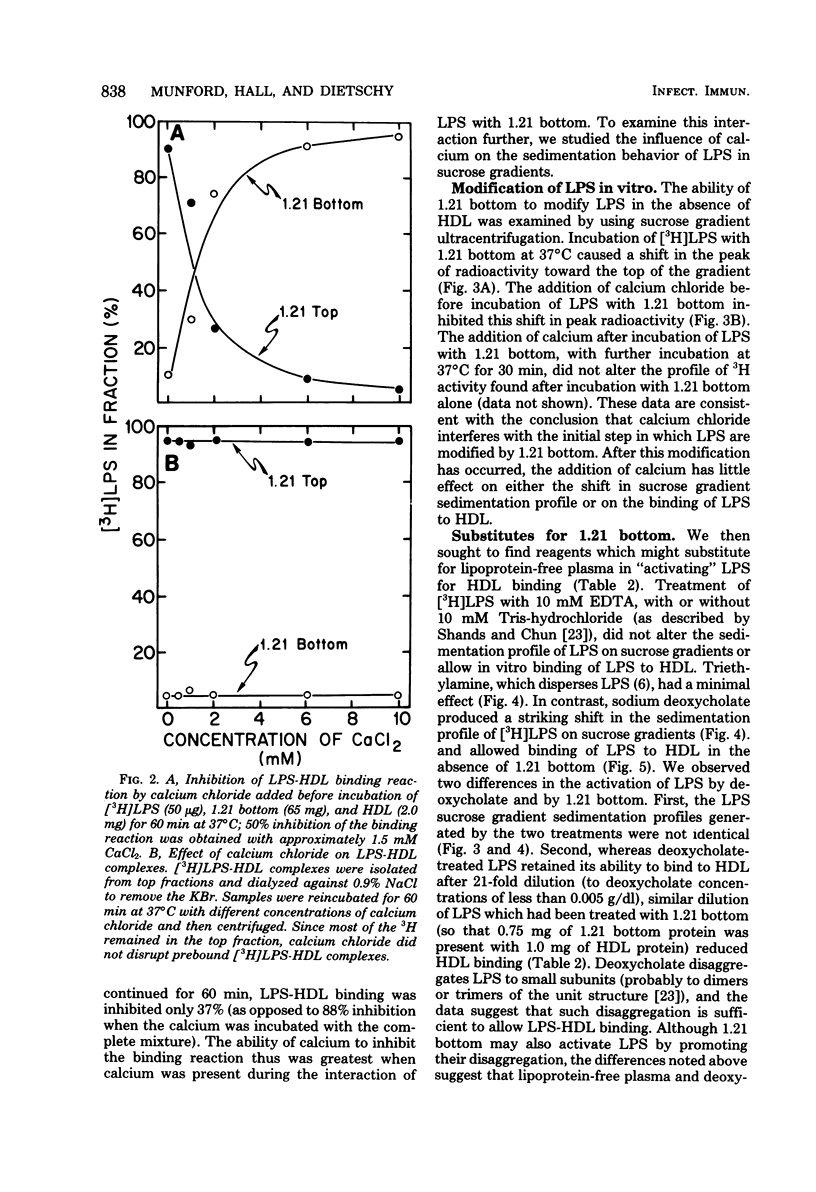
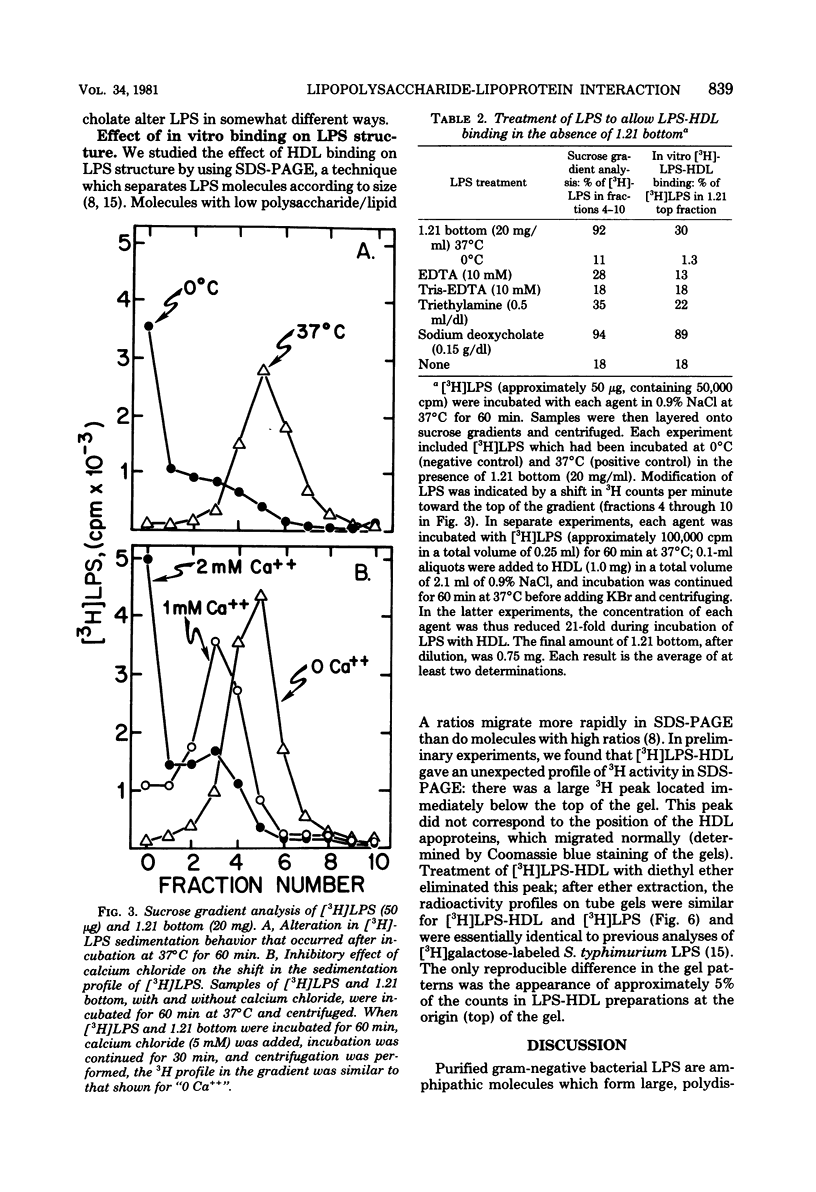
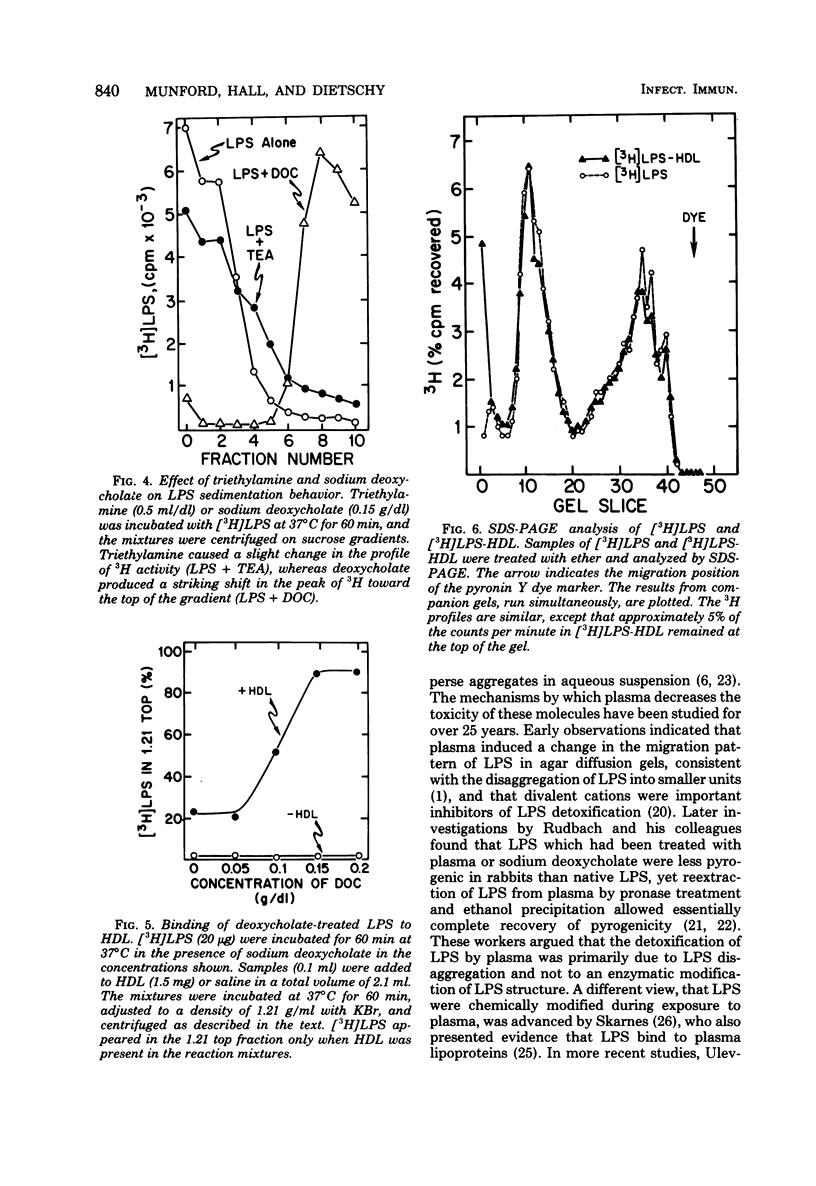
These studies were undertaken to investigate the binding of gram-negative bacterial lipopolysaccharides (LPS) to high-density lipoproteins (HDL) of rat plasma. Purified Salmonella typhimurium LPS, intrinsically labeled with [3H]-galactose, bound rapidly in vitro to isolated rat HDL. Maximal binding of LPS to HDL occurred when LPS and HDL were incubated with lipoprotein-free plasma (rho greater than 1.21 g/ml). Since LPS, when purified, form large aggregates, we tested the hypothesis that disaggregation of LPS enhances LPS-HDL binding. We found that calcium chloride (1 mM), an agent which prevents LPS disaggregation, inhibited binding of LPS to HDL by interfering with the modification of LPS by lipoprotein-free plasma. Conversely, sodium deoxycholate (0.15 g/dl), which disaggregates LPS, greatly increased binding of LPS to HDL in the absence of lipoprotein-free plasma. Analysis of labeled LPS by sodium deodecyl sulfate-polyacrylamide gel electrophoresis showed only minor differences in the sizes of LPS molecules before and after binding to HDL, suggesting that chemical modification of LPS is not required for binding. The results provide evidence that disaggregation increases the binding of LPS to HDL.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLUFF L. E. A study of the effect of serum on the immunological reaction of a bacterial endotoxin. J Exp Med. 1956 Apr 1;103(4):439–452. doi: 10.1084/jem.103.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Wolff S. M. Biology of endotoxin. Annu Rev Med. 1976;27:127–141. doi: 10.1146/annurev.me.27.020176.001015. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Filkins J. P. Bioassay of endotoxin inactivation in the lead-sensitized rat. Proc Soc Exp Biol Med. 1970 Jul;134(3):610–612. doi: 10.3181/00379727-134-34844. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Bøg-Hansen T. C., Back U., Galanos C. Interaction of lipopolysaccharides with plasma high-density lipoprotein in rats. Infect Immun. 1980 May;28(2):373–380. doi: 10.1128/iai.28.2.373-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Goralnick S., Osborn M. J. Isolation from human serum of an inactivator of bacterial lipopolysaccharide. Am J Pathol. 1977 Sep;88(3):559–574. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasser N. L., Roheim P. S., Edelstein D., Eder H. A. Serum lipoproteins of normal and cholesterol-fed rats. J Lipid Res. 1973 Jan;14(1):1–8. [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J Immunol. 1979 Nov;123(5):2133–2143. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Warner R. C. Physicochemical studies on a lipopolysaccharide from the cell wall of Azotobacter vinelandii. J Biol Chem. 1967 Nov 10;242(21):4994–5001. [PubMed] [Google Scholar]
- ROSEN F. S., SKARNES R. C., LANDY M., SHEAR M. J. Inactivation of endotoxin by a humoral component. III. Role of divalent cation and a dialyzable component. J Exp Med. 1958 Nov 1;108(5):701–711. doi: 10.1084/jem.108.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Girard A., Rothfield L. Reconstitution of a functional membrane enzyme system in a monomolecular film. I. Formation of a mixed monolayer of lipopolysaccharide and phospholipid. J Mol Biol. 1970 Nov 14;53(3):475–490. doi: 10.1016/0022-2836(70)90078-1. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A., Anacker R. L., Haskins W. T., Johnson A. G., Milner K. C., Ribi E. Physical aspects of reversible inactivation of endotoxin. Ann N Y Acad Sci. 1966 Jun 30;133(2):629–643. doi: 10.1111/j.1749-6632.1966.tb52394.x. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A., Johnson A. G. Alteration and restoration of endotoxin activity after complexing with plasma proteins. J Bacteriol. 1966 Oct;92(4):892–898. doi: 10.1128/jb.92.4.892-898.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKARNES R. C., ROSEN F. S., SHEAR M. J., LANDY M. Inactivation of endotoxin by a humoral component. II. Interaction of endotoxin with serum and plasma. J Exp Med. 1958 Nov 1;108(5):685–699. doi: 10.1084/jem.108.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W., Jr, Chun P. W. The dispersion of gram-negative lipopolysaccharide by deoxycholate. Subunit molecular weight. J Biol Chem. 1980 Feb 10;255(3):1221–1226. [PubMed] [Google Scholar]
- Skarnes R. C. Host defense against bacterial endotoxemia: mechanism in normal animals. J Exp Med. 1970 Aug 1;132(2):300–316. doi: 10.1084/jem.132.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes R. C. In vivo interaction of endotoxin with a plasma lipoprotein having esterase activity. J Bacteriol. 1968 Jun;95(6):2031–2034. doi: 10.1128/jb.95.6.2031-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]


