Abstract
Natural compounds that pose no significant medical or environmental side effects are potential sources of antifungal agents, either in their nascent form or as structural backbones for more effective derivatives. Kojic acid (KA) is one such compound. It is a natural by-product of fungal fermentation commonly employed by food and cosmetic industries. We show that KA greatly lowers minimum inhibitory (MIC) or fungicidal (MFC) concentrations of commercial medicinal and agricultural antifungal agents, amphotericin B (AMB) and strobilurin, respectively, against pathogenic yeasts and filamentous fungi. Assays using two mitogen-activated protein kinase (MAPK) mutants, i.e., sakAΔ, mpkCΔ, of Aspergillus fumigatus, an agent for human invasive aspergillosis, with hydrogen peroxide (H2O2) or AMB indicate such chemosensitizing activity of KA is most conceivably through disruption of fungal antioxidation systems. KA could be developed as a chemosensitizer to enhance efficacy of certain conventional antifungal drugs or fungicides.
Keywords: Kojic acid, hydrogen peroxide, amphotericin B, strobilurin, chemosensitization
1. Introduction
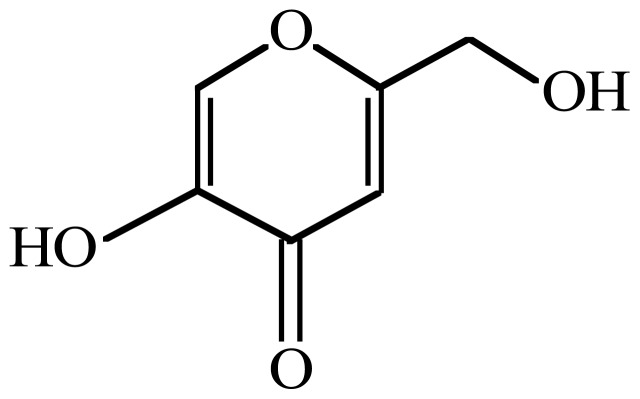
Kojic acid (KA, Figure 1) is a natural pyrone produced by certain filamentous fungi, mainly species of Aspergillus and Penicillium. It is a common by-product in the fermentation of soy sauce, sake and rice wine, and is widely used as a food additive to prevent oxidative browning, or in cosmetics as a depigmenting agent [1–3]. Genes involved in KA biosynthesis were recently identified [4,5]. Cellular immunity is enhanced by KA through stimulating phagocytosis and generation of reactive oxygen species (ROS) in macrophages, and potentiation of phytohemagglutinin-based proliferation of lymphocytes [6,7]. KA is fungistatic against the pathogenic yeast, Cryptococcus neoformans, by inhibiting melanin production required for infectivity [8]. Derivatives of KA also have antimicrobial activity against a variety of other fungi and bacteria [9], showing its potential as a polyfunctional backbone for new antimicrobial agents [10].
Figure 1.
Structure of kojic acid (KA).
Among Aspergillus species, A. flavus, A. parasiticus and A. oryzae are the main producers of KA [11]. A. oryzae is used widely in the food industry. However, A. flavus and A. parasiticus are opportunistic pathogens of various crops, and a concern since they produce carcinogenic aflatoxins that can contaminate food. A. flavus is also an agent for human invasive aspergillosis (IA). Of note, the chief agent of IA, A. fumigatus, and a third IA agent, A. terreus, do not produce KA [12–14].
Co-application of certain types of compounds can enhance efficacy of conventional antimicrobial agents through a process termed “chemosensitization.” With regard to microbial pathogens, a chemosensitizer functions by debilitating the ability of a pathogen to completely activate a defense mechanism to an antimicrobial agent [15,16]. We investigated if KA, as a chemosensitizer, could improve activity of commercial antifungal agents against pathogenic strains of Aspergillus and yeasts (See Table 1). We tested this chemosensitizing potential by co-applying KA with hydrogen peroxide (H2O2) to mimic host ROS, and with a commercial antimycotic, amphotericin B (AMB) and agricultural fungicides, fludioxonil (FLUD) and strobilurin (kresoxim methyl (Kre-Me)).
Table 1.
Fungal strains used in this study.
| Fungal strains | Strain characteristics | Source/Reference |
|---|---|---|
| Filamentous fungi | ||
|
| ||
| Aspergillus flavus 3357 | Kojic acid producer, Human pathogen (aspergillosis), Plant pathogen | NRRL a |
| A. parasiticus 5862 | Kojic acid producer, Plant pathogen | NRRL a |
| A. fumigatus AF293 | Human pathogen (aspergillosis), Reference clinical strain | [17] |
| A. fumigatus sakAΔ | Human pathogen (aspergillosis), MAPK mutant derived from AF293 | [17] |
| A. fumigatus mpkCΔ | Human pathogen (aspergillosis), MAPK mutant derived from AF293 | [18] |
| A. terreus UAB673 | Human pathogen (aspergillosis), Clinical isolate | CDC b |
| A. terreus UAB680 | Human pathogen (aspergillosis), Clinical isolate | CDC b |
| A. terreus UAB698 | Human pathogen (aspergillosis), Clinical isolate | CDC b |
|
| ||
| Yeasts | ||
|
| ||
| Candida albicans 90028 | Human pathogen (candidiasis), Reference clinical strain | ATCC c |
| C. albicans CAN276 | Human pathogen (candidiasis), Clinical isolate | IHMT d |
| C. krusei 6258 | Human pathogen (candidiasis), Reference clinical strain | ATCC c |
| C. krusei CAN75 | Human pathogen (candidiasis), Clinical isolate | IHMT d |
| C. tropicalis CAN286 | Human pathogen (candidiasis), Clinical isolate | IHMT d |
| Cryptococcus neoformans CN24 | Human pathogen (cryptococcosis), Clinical isolate | IHMT d |
| Saccharomyces cerevisiae BY4741 | Model yeast, Parental strain (Mat a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) | SGD e |
| S. cerevisiae bck1Δ | MAPK mutant derived from BY4741 | SGD e |
| S. cerevisiae slt2Δ | MAPK kinase kinase mutant derived from BY4741 | SGD e |
NRRL, National Center for Agricultural Utilization and Research, USDA-ARS, Peoria, IL, USA.
CDC, Centers for Disease Control and Prevention, Atlanta, GA, USA.
ATCC, American Type Culture Collection, Manassas, VA, USA.
IHMT, Instituto de Higiene e Medicina Tropical/CREM, Universidade Nova de Lisboa, Portugal.
SGD, Saccharomyces Genome Database [19].
2. Results and Discussion
2.1. Enhanced Antimycotic Activity of H2O2 by KA against Filamentous Fungi
2.1.1. Agar Plate Bioassay: Filamentous Fungi
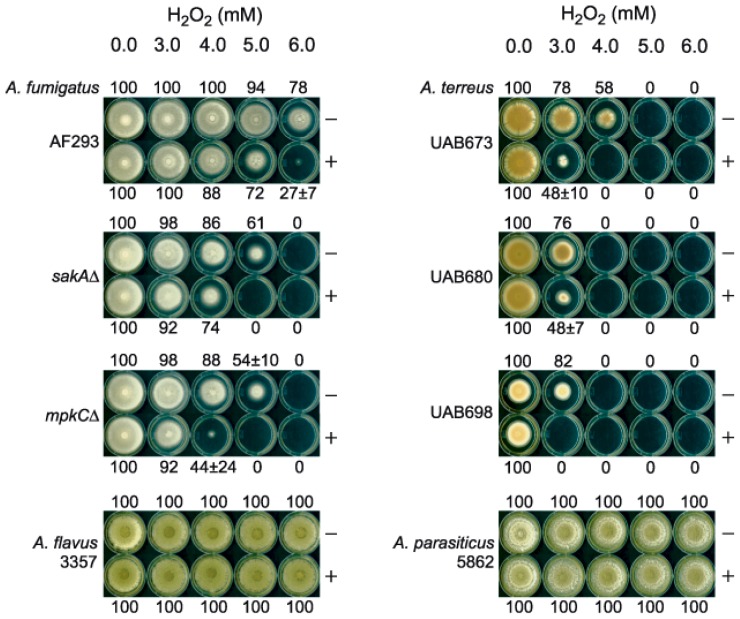
We initially tested KA (5 mM) and H2O2 (3, 4, 5, 6 mM) on filamentous fungal growth, comparing colony diameter to controls in agar bioassays (See Experimental Section). Three strains of A. fumigatus (wild type strain, AF293, and two deletion mutants for oxidative/osmotic stress responsive mitogen-activated protein kinase (MAPK), sakAΔ and mpkCΔ) [17,18], three clinical strains of A. terreus (UAB-673, −680 and −698), and one wild type strain, each, of A. flavus (NRRL3357) and A. parasiticus (NRRL5862), were tested. Fungi were cultured at 35 °C, except A. parasiticus at 28 °C, on potato dextrose agar (PDA).
Results showed (Figure 2): (1) KA (at 5 mM) did not affect growth of any strain; (2) H2O2 (up to 6 mM) alone or with KA had no effect on A. flavus or A. parasiticus; (3) H2O2 alone or with KA inhibited growth of all strains of A. fumigatus and A. terreus. Strain sensitivity to KA + H2O2 varied as follows (in decreasing order): A. terreus UAB698 > strains 680 = 673 > A. fumigatus mpkCΔ = sakAΔ > AF293 > A. flavus = A. parasiticus. Therefore, KA + H2O2 treatments inhibited growth much more significantly in strains that do not produce KA (i.e., A. fumigatus, A. terreus).
Figure 2.
Agar bioassay showing antifungal chemosensitization of kojic acid (KA) with H2O2 tested against Aspergillus strains. Numbers (0–100) indicate percent (%) radial growth compared to non-treated control (100%; no H2O2 and no KA). (−), w/o KA; (+), w/KA (5 mM).
2.1.2. Microtiter Plate (microdilution) Bioassay: Filamentous Fungi
Based on results of the agar bioassay (shown above), antifungal interactions between KA and H2O2 were assessed further for only the A. fumigatus and A. terreus strains using triplicate, microtiter-plate checkerboard bioassays (Clinical Laboratory Standards Institute (CLSI) M38-A) [20] with concentration ranges of KA, 0.2–12.8 mM, and H2O2, 0.0625–16 mM (See Experimental Section).
Minimum inhibitory concentrations (MICs), lowest concentration of agent(s) showing no visible fungal growth, were assessed after 48 h. Minimum fungicidal concentrations (MFCs), lowest concentration of agents showing ≥99.9% fungal death, were determined (following completion of MIC assays) wherein entire volumes of microtiter wells (200 μL) were spread onto individual PDA plates, and cultured for another 48 h. Compound interactions, Fractional Inhibitory Concentration Indices (FICI) and Fractional Fungicidal Concentration Indices (FFCI) were calculated, as follows: FICI or FFCI = (MIC or MFC of compound A in combination with compound B/MIC or MFC of compound A, alone) + (MIC or MFC of compound B in combination with compound A/MIC or MFC of compound B, alone). Interactions were defined as: “synergistic” (FICI or FFCI ≤ 0.5) or “indifferent” (FICI or FFCI > 0.5–4) [21].
Synergistic FICIs and FFCIs between KA and H2O2 only occurred in AF293. Despite the absence of calculated “synergism” as depicted by “indifferent” interactions (by definition) (Table 2), there was enhanced antifungal activity (i.e., chemosensitization) in the remaining A. fumigatus and A. terreus strains. This enhancement was indicated by lower MICs and MFCs for either or both KA and H2O2 when co-applied. Also, the A. fumigatus MAPK mutants had half the MICs and MFCs of AF293 (Table 2; Figure 3a), suggesting that, in the wild type fungi, MAPKs in the oxidative/osmotic stress responsive pathway play protective roles against the antimycotic activity of KA + H2O2.
Table 2.
Antifungal chemosensitization of kojic acid (mM) with H2O2 (mM) tested against Aspergillus strains. a Minimum fungicidal concentrations (MFCs) are concentrations where ≥99.9% fungal death was achieved.
| Strains | Compounds | MIC alone | MIC combined | FICI | MFC alone | MFC combined | FFCI |
|---|---|---|---|---|---|---|---|
| A. fumigatus | Kojic | >12.8 b | 0.8 | 0.5 | >12.8 | 0.8 | 0.5 |
| AF293 | H2O2 | 8 | 4 | 8 | 4 | ||
| A. fumigatus | Kojic | >12.8 | 12.8 | 1.0 | >12.8 | 12.8 | 1.0 |
| sakAΔ | H2O2 | 4 | 2 | 4 | 2 | ||
| A. fumigatus | Kojic | >12.8 | 12.8 | 1.0 | >12.8 | 12.8 | 1.0 |
| mpkCΔ | H2O2 | 4 | 2 | 4 | 2 | ||
| A. terreus | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | 12.8 | 0.8 |
| UAB673 | H2O2 | 2 | 1 | 4 | 1 | ||
| A. terreus | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | 12.8 | 1.0 |
| UAB680 | H2O2 | 2 | 1 | 2 | 1 | ||
| A. terreus | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | 12.8 | 1.0 |
| UAB698 | H2O2 | 2 | 1 | 2 | 1 | ||
|
| |||||||
| Mean | Kojic | >12.8 | 7.6 | 0.8 | >12.8 | 10.8 | 0.9 |
| H2O2 | 3.7 | 1.8 | 4.0 | 1.8 | |||
|
| |||||||
| t-test | Kojic | - | p < 0.001 | - | - | p < 0.001 | - |
| H2O2 | p < 0.5 | p < 0.1 | |||||
MIC: Minimum inhibitory concentration, MFC: Minimum fungicidal concentration, FICI: Fractional Inhibitory Concentration Indices, FFCI: Fractional Fungicidal Concentration Indices. Student’s t-test for paired data (combined, i.e., chemosensitization) was vs. mean MIC or MFC of each compound (alone, i.e., no chemosensitization) determined in six strains. Calculation was based on [22].
Kojic acid was tested up to 12.8 mM. For calculation purpose, 25.6 mM (doubling of 12.8 mM) was used.
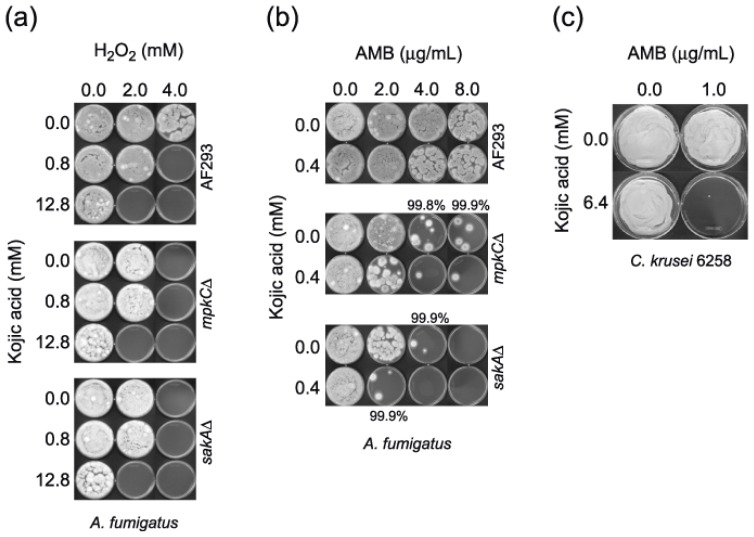
Figure 3.
(a) MFC determination of A. fumigatus strains (AF293, sakAΔ, mpkCΔ) with the treatment of kojic acid (KA) + H2O2. (b) MFC determination in A. fumigatus sakAΔ strain with the treatment of KA + AMB. Results indicated that A. fumigatus AF293 and mpkCΔ strains needed higher concentration of KA or AMB to achieve ≥99.9% cell death compared to sakAΔ. (c) MFC determination in Candida krusei ATCC6258 with the treatment of KA + AMB.
2.2. Enhanced Antimycotic Activity of AMB with KA in Filamentous Fungi and Yeasts
AMB is an antimycotic drug against filamentous or yeast pathogens. However, AMB can be associated with significant side effects resulting in nephrosis and other tissue-damage in invasive pulmonary aspergillosis [23]. Therefore, we reasoned that use of chemosensitizing agents from natural sources could enhance the effectiveness of AMB, while lowering toxicity of this polyene drug to human cells. The main mode of action of AMB is disruption of the fungal plasma membrane, resulting in ion leakage. However, AMB also induces oxidative damage [24–27] by stimulating ROS production [28]. Since KA contributed to oxidative stress when combined with H2O2 in Aspergillus (See Table 2), we surmised it might also enhance AMB activity.
2.2.1. Microtiter Plate (microdilution) Bioassay: Filamentous Fungi
Checkerboard assays of KA (0.2–12.8 mM) and AMB (0.125–32 μg/mL) (See Experimental Section) were initially used to assess antifungal interactions against the Aspergillus strains, by using CLSI M38-A protocol [20]. In assays of the Aspergillus strains, co-application of KA increased AMB activity only in strains of A. fumigatus, where FICIs and FFCIs were synergistic in the A. fumigatus MAPK mutant strains (Table 3; Figure 3b).
Table 3.
Antifungal chemosensitization of kojic acid (mM) with AMB (μg/mL) tested against Aspergillus and yeast strains. a MFCs are concentrations where ≥99.9% fungal death was achieved, except where noted in the Table.
| Strains | Compounds | MIC alone | MIC combined | FICI | MFC alone | MFC combined | FFCI |
|---|---|---|---|---|---|---|---|
| A. fumigatus | Kojic | >12.8 b | 0.8 | 0.8 | >12.8 | 3.2 | 0.6 |
| AF293 | AMB | 4 | 2 | >32 c | 32 | (99.8% inhibition) | |
| A. fumigatus | Kojic | >12.8 | 12.8 | 1.0 | >12.8 | 0.4 | 0.5 |
| sakAΔ | AMB | 2 | 1 | (85%–90% inhibition) | 4 | 2 | |
| A. fumigatus | Kojic | >12.8 | 0.2 | 0.5 | >12.8 | 0.2 | 0.5 |
| mpkCΔ | AMB | 4 | 2 | 8 | 4 | ||
| C. albicans | Kojic | >12.8 | 6.4 | 0.8 | >12.8 | > 12.8 | 2.0 |
| CAN276 | AMB | 1 | 0.5 | 1 | 1 | ||
| C. krusei | Kojic | >12.8 | 0.4 | 0.5 | >12.8 | 6.4 | 0.8 |
| ATCC 6258 | AMB | 2 | 1 | 2 | 1 | ||
| Cryptococcus | Kojic | >12.8 | 0.4 | 0.5 | >12.8 | 3.2 | 0.6 |
| neoformans CN24 | AMB | 2 | 1 | 2 | 1 | ||
|
| |||||||
| Mean | Kojic | >12.8 | 3.5 | 0.7 | >12.8 | 6.5 | 0.8 |
| AMB | 2.5 | 1.3 | 13.5 | 6.8 | |||
|
| |||||||
| t-test | Kojic | - | p < 0.001 | - | - | p < 0.005 | - |
| AMB | p < 0.05 | p < 1.0 | |||||
AMB: Amphotericin B. MIC: Minimum inhibitory concentration, MFC: Minimum fungicidal concentration. FICI: Fractional Inhibitory Concentration Indices, FFCI: Fractional Fungicidal Concentration Indices. Student’s t-test for paired data (combined, i.e., chemosensitization) was vs. mean MIC or MFC of each compound (alone, i.e., no chemosensitization) determined in six strains. Calculation was based on [22].
Kojic acid was tested up to 12.8 mM. For calculation purpose, 25.6 mM (doubling of 12.8 mM) was used.
AMB was tested up to 32 μg/mL. For calculation purpose, 64 μg/mL (doubling of 32 μg/mL) was used.
2.2.2. Microtiter Plate (microdilution) Bioassay: Yeasts
Checkerboard assays of the yeast strains employed methods outlined in the European Committee on Antimicrobial Susceptibility Testing (EUCAST)] [29]. According to these methods, MICs were determined at 24 h for Candida and Saccharomyces, and at 48 h for Cryptococcus. Following MIC determinations, MFCs were determined on Yeast Peptone Dextrose (YPD) agar, where cells were cultured for an additional 48 h for Candida/Saccharomyces or 72 h for Cryptococcus, respectively.
Among the Candida and Cryptococcus strains tested, KA enhanced AMB activity in C. albicans CAN276, C. krusei ATCC6258, C. neoformans CN24 (Table 3). Synergism of KA + AMB was observed in C. krusei ATCC6258 and C. neoformans strains (Table 3; Figure 3c).
In parallel checkerboard assays of S. cerevisiae, the wild type and two MAPK cell wall integrity mutant strains, i.e., slt2Δ (MAPK deletion; cell wall integrity pathway) and bck1Δ (MAPK kinase kinase deletion; cell wall integrity pathway) were included. We tried to determine whether the MAPK system for cell wall integrity plays a protective role against the antimycotic activity of KA + AMB. These mutants previously showed hypersensitivity to certain environmental stresses [30,31]. However, the mutants were not more sensitive than the wild type to co-application of either compound (Table 4), indicating Slt2p and Bck1p (viz., cell wall integrity pathway) do not participate in yeast cell homeostasis under KA + AMB treatment.
Table 4.
Antifungal chemosensitization of kojic acid (mM) with AMB (μg/mL). a MFCs are concentrations where ≥99.9% fungal death was achieved.
| Strains | Compounds | MIC alone | MIC combined | FICI | MFC alone | MFC combined | FFCI |
|---|---|---|---|---|---|---|---|
| S. cerevisiae | Kojic | >12.8 b | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| BY4741 | AMB | 2 | 1 | 4 | 2 | ||
| S. cerevisiae | Kojic | >12.8 | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| slt2Δ | AMB | 2 | 1 | 4 | 2 | ||
| S. cerevisiae | Kojic | >12.8 | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| bck1Δ | AMB | 2 | 1 | 4 | 2 | ||
|
| |||||||
| Mean | Kojic | >12.8 | 6.4 | 0.8 | > 12.8 | 12.8 | 1.0 |
| AMB | 2 | 1 | 4 | 2 | |||
|
| |||||||
| t-test | Kojic | - | p < 0.001 | - | - | p < 0.001 | - |
| AMB | p < 0.001 | p < 0.001 | |||||
AMB: Amphotericin B. MIC: Minimum inhibitory concentration, MFC: Minimum fungicidal concentration. FICI: Fractional Inhibitory Concentration Indices, FFCI: Fractional Fungicidal Concentration Indices. Student’s t-test for paired data (combined, i.e., chemosensitization) was vs. mean MIC or MFC of each compound (alone, i.e., no chemosensitization) determined in three strains. Calculation was based on [22].
Kojic acid was tested up to 12.8 mM. For calculation purpose, 25.6 mM (doubling of 12.8 mM) was used.
2.3. No Enhancement of Antimycotic Activity of H2O2 with KA in Yeasts
KA (5 mM) and H2O2 (2 and 3 mM) co-application was tested against yeast in agar bioassays, including five clinical strains of Candida, one of C. neoformans and non-pathogenic, S. cerevisiae. Yeast cells (1 × 106) were serially diluted (10-fold), spotted onto Synthetic Glucose (SG) agar incorporated with KA and/or H2O2, and incubated at 30 °C, S. cerevisiae, or 35 °C, Candida/Cryptococcus (See [32] for methods). These assays revealed no effect (data not shown) and hence, checkerboard assays to determine MICs, FICIs, etc., were not performed.
The results of all chemosensitization tests (i.e., KA + H2O2 or AMB in filamentous and yeast strains) are summarized in Table 5.
Table 5.
Summary of responses of Aspergillus and yeast strains to the co-application of kojic acid with H2O2 or AMB. a
| Fungal strains | Agents co-applied | |
|---|---|---|
|
| ||
| H2O2 (FICI, FFCI) b | AMB (FICI, FFCI) b | |
| Filamentous fungi | ||
|
| ||
| Aspergillus flavus 3357 | - | - |
| A. parasiticus 5862 | - | - |
| A. fumigatus AF293 | + (0.5, 0.5) | + (0.8, 0.6) |
| A. fumigatus sakAΔ | + (1.0, 1.0) | + (1.0, 0.5) |
| A. fumigatus mpkCΔ | + (1.0, 1.0) | + (0.5, 0.5) |
| A. terreus UAB673 | + (0.8, 0.8) | - |
| A. terreus UAB680 | + (0.8, 1.0) | - |
| A. terreus UAB698 | + (0.8, 1.0) | - |
|
| ||
| Yeasts | ||
|
| ||
| Candida albicans 90028 | - | - |
| C. albicans CAN276 | - | + (0.8, 2.0) |
| C. krusei 6258 | - | + (0.5, 0.8) |
| C. krusei CAN75 | - | - |
| C. tropicalis CAN286 | - | - |
| Cryptococcus neoformans CN24 | - | + (0.5, 0.6) |
| Saccharomyces cerevisiae BY4741 | - | + (0.8, 1.0) |
| S. cerevisiae bck1Δ | - | + (0.8, 1.0) |
| S. cerevisiae slt2Δ | - | + (0.8, 1.0) |
+, enhancement of antifungal activity after co-application; -, no enhancement of antifungal activity after co-application.
2.4. Enhanced Antimycotic Activity of Strobilurin with KA in A. fumigatus
We also tested combinations of KA with agricultural fungicides, fludioxonil (FLUD) or Kre-Me (strobilurin), fungicides that target different components of the oxidative stress response system [33,34], by using A. fumigatus wild type and MAPK (sakAΔ, mpkCΔ) mutants. Certain fungi with mutations in genes involved in signal transduction of stress response, e.g., MAPK signaling pathway, can escape toxicity of the commercial fungicide FLUD [34]. In a prior study we found redox-active benzo derivatives co-applied with either of these fungicides reduced effective dosages and prevented tolerance of A. fumigatus sakAΔ and mpkCΔ mutants to FLUD [35]. However, in our present study, co-application of KA with FLUD did not overcome tolerance of these mutants to this fungicide (Figure 4a).
Figure 4.
(a) Agar bioassay showing co-application of kojic acid (KA) could not overcome the tolerance of Aspergillus fumigatus sakAΔ and mpkCΔ mutants to fludioxonil (FLUD). None, no treatment control; FLUD 50 μM; KA 30 mM. (b) Agar bioassay showing co-application of KA enhanced the antifungal activity of strobilurin (Kre-Me) in A. fumigatus strains. None, no treatment control; Kre-Me 25 μM; KA 25 mM.
In a parallel study, we tested combinations of KA with Kre-Me. Kre-Me is an inhibitor of complex III of the mitochondrial respiratory chain (MRC), the key route system for cellular energy (ATP) production [36]. Moreover, disruption of complex III of the MRC results in an abnormal release of electrons that additionally cause cellular oxidative stress [37]. Therefore, antioxidant enzymes play important roles in protecting cells from oxidative damage triggered by MRC inhibitors. KA improved antimycotic activity of Kre-Me against all A. fumigatus strains (Figure 4b), where A. fumigatus sakAΔ and mpkCΔ mutants showed relatively higher tolerance to Kre-Me than the wild type (AF293). Thus, results indicated that the chemosensitizing mechanism of KA might not involve glutathione/superoxide dismutase-based oxidative stress response, differing from redox-active benzo derivatives [35]. We speculated that, in addition to inhibiting ATP production, co-application of KA and Kre-Me might involve responses of other types of antioxidant enzymes/systems. Comprehensive chemosensitization tests using KA with additional strobilurins are currently underway in various filamentous fungi, including Aspergillus, Penicillium, Acremonium, Scedosporium, and others (Note: There was no chemosensitization effect of KA with any azole drug, such as fluconazole, ketoconazole, itraconazole, in Aspergillus or yeasts (data not shown)).
3. Experimental Section
3.1. Fungal Strains and Culture Conditions
Aspergillus strains (See Table 1) were grown at 35 °C on potato dextrose agar (PDA; Sigma, St. Louis, MO, USA), except A. parasiticus, which was grown at 28 °C on PDA. Yeast strains (Candida albicans, C. krusei, C. tropicalis, Cryptococcus neoformans, Saccharomyces cerevisiae; See Table 1) were cultured on Synthetic Glucose (SG; Yeast nitrogen base without amino acids 0.67%, glucose 2% with appropriate supplements: uracil 0.02 mg/mL, amino acids 0.03 mg/mL) or Yeast Peptone Dextrose (YPD; Bacto yeast extract 1%, Bacto peptone 2%, glucose 2%) medium at 35 °C for yeast pathogens (Candida, Cryptococcus) or 30 °C for S. cerevisiae, respectively.
3.2. Chemicals
Antifungal chemosensitizing agent (kojic acid (KA)), antifungal drugs (amphotericin B (AMB), fluconazole, ketoconazole, itraconazole), strobilurin (kresoxim methyl (Kre-Me)) and oxidizing agent (hydrogen peroxide (H2O2)) were procured from Sigma Co. (St. Louis, MO, USA). Each compound was dissolved in dimethyl sulfoxide (DMSO; absolute DMSO amount: <1% in media), except H2O2, which was dissolved in water, before incorporation into culture media. In all tests, control plates (i.e., “No treatment”) contained DMSO at levels equivalent to that of cohorts receiving antifungal agents, within the same set of experiments.
3.3. Antifungal Bioassay
3.3.1. Agar Plate Bioassay: Filamentous Fungi
In the plate bioassay, measurement of sensitivities of filamentous fungi to the antifungal agents was based on percent (%) radial growth of treated compared to control (“No treatment”) fungal colonies (See text for test concentrations.) [38]. Minimum inhibitory concentration (MIC) values on agar plates were determined based on triplicate bioassays, and defined as the lowest concentration of agents where no fungal growth was visible on the plate. For the above assays, fungal conidia (5 × 104 CFU/mL) were diluted in phosphate-buffered saline (PBS) and applied as a drop onto the center of PDA plates with or without antifungal compounds. Growth was observed for three to seven days to determine cellular sensitivities to drugs/compounds.
3.3.2. Microtiter Plate (microdilution) Bioassay: Filamentous Fungi
To determine antifungal chemosensitizing activities of KA (0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8 mM) to antifungal drug (AMB; 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32 μg/mL) or H2O2 (0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16 mM) in filamentous fungi, checkerboard bioassays (0.4 × 104–5 × 104 CFU/mL) were performed in microtiter wells using a broth microdilution (with RPMI 1640 medium; Sigma Co. (St. Louis, MO, USA), according to methods outlined by the Clinical Laboratory Standards Institute (CLSI) M38-A [20]. MICs for chemosensitization were defined as the concentrations where no fungal growth was visible at 48 and 72 h. All bioassays were performed in triplicate. Statistical analysis was based on [22].
3.3.3. Microtiter Plate (microdilution) Bioassay: Yeasts
Chemosensitizing activities of KA (0.2, 0.4, 0.8, 1.6, 3.2, 6.4, 12.8 mM) to antifungal drug (AMB; 0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32 μg/mL) or H2O2 (0.0625, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16 mM) were determined by using checkerboard bioassays in microtiter plates (with RPMI 1640 medium, except SG for S. cerevisiae; Sigma Co., Madrid, Spain). To determine changes in MICs of antifungal agents (i.e., drugs and chemosensitizers) in microtiter wells, checkerboard bioassays (0.5 × 105 to 2.5 × 105 CFU/mL) were performed using broth microdilution protocols according to methods outlined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [29]. MICs for chemosensitization were defined as the concentrations where no fungal growth was visible at 24 and 48 h. All bioassays were performed in triplicate. Statistical analysis was based on [22].
4. Conclusions
In summary, enhancing antifungal interactions of KA in combination with H2O2, AMB, FLUD or Kre-Me were, as follows: (1) All A. fumigatus strains were sensitive to either KA + H2O2 or KA + AMB; (2) A. terreus strains were only sensitive to KA + H2O2; (3) C. albicans CAN276, C. krusei ATCC6258, C. neoformans CN24, S. cerevisiae were only sensitive to KA + AMB; and (4) A. flavus 3357, A. parasiticus 5862, C. albicans 90028, C. krusei CAN75, C. tropicalis CAN286 were marginally or not sensitive to any co-treatments; (5) A. fumigatus AF293 was more sensitive than the MAPK mutant strains to KA + Kre-Me. Thus, the antifungal chemosensitizing capacity of KA appears to be antifungal agent and/or fungal strain-specific. In conclusion, KA, a safe natural compound, may have a new use as an enhancer of certain commercial antifungal agents, such as AMB, H2O2 or strobilurin, against defined fungal pathogens. The enhancing effect appears to involve the modulation of the function of oxidative stress response system in the fungus. Further studies are warranted to determine the precise mechanism of action of KA for antifungal chemosensitization.
Acknowledgments
We thank Gregory S. May at The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA, for providing Aspergillus fumigatus (AF293, sakAΔ and mpkCΔ mutants) strains and Arun Balajee, Centers for Disease Control and Prevention, Atlanta, GA, USA, for the strains of A. terreus. This research was conducted under USDA-ARS CRIS Project 5325-42000-037-00D.
References
- 1.Bentley R. From miso, saké and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006;23:1046–1062. doi: 10.1039/b603758p. [DOI] [PubMed] [Google Scholar]
- 2.Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leyden J.J., Shergill B., Micali G., Downie J., Wallo W. Natural options for the management of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2011;25:1140–1145. doi: 10.1111/j.1468-3083.2011.04130.x. [DOI] [PubMed] [Google Scholar]
- 4.Terabayashi Y., Sano M., Yamane N., Marui J., Tamano K., Sagara J., Dohmoto M., Oda K., Ohshima E., Tachibana K., et al. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol. 2010;47:953–961. doi: 10.1016/j.fgb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Oda K., Kobayashi A., Ohashi S., Sano M. Aspergillus oryzae laeA regulates kojic acid synthesis genes. Biosci. Biotechnol. Biochem. 2011;75:1832–1834. doi: 10.1271/bbb.110235. [DOI] [PubMed] [Google Scholar]
- 6.Niwa Y., Akamatsu H. Kojic acid scavenges free radicals while potentiating leukocyte functions including free radical generation. Inflammation. 1991;15:303–315. doi: 10.1007/BF00917315. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues A.P., Carvalho A.S., Santos A.S., Alves C.N., do Nascimento J.L., Silva E.O. Kojic acid, a secondary metabolite from Aspergillus sp., acts as an inducer of macrophage activation. Cell Biol. Int. 2011;35:335–343. doi: 10.1042/CBI20100083. [DOI] [PubMed] [Google Scholar]
- 8.Chee H.Y., Lee E.H. Fungistatic activity of kojic acid against human pathogenic fungi and inhibition of melanin-production in Cryptococcus neoformans. Mycobiology. 2003;31:248–250. [Google Scholar]
- 9.Reddy B.V., Reddy M.R., Madan C.H., Kumar K.P., Rao M.S. Indium(III) chloride catalyzed three-component coupling reaction: A novel synthesis of 2-substituted aryl(indolyl)kojic acid derivatives as potent antifungal and antibacterial agents. Bioorg. Med. Chem. Lett. 2010;20:7507–7511. doi: 10.1016/j.bmcl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Brtko J., Rondahl L., Ficková M., Hudecová D., Eybl V., Uher M. Kojic acid and its derivatives: History and present state of art. Cent. Eur. J. Public Health. 2004;12:S16–S18. [PubMed] [Google Scholar]
- 11.Mohamad R., Mohamed M.S., Suhaili N., Salleh M.M., Ariff A.B. Kojic acid: Applications and development of fermentation process for production. Biotech. Mol. Biol. Rev. 2010;5:24–37. [Google Scholar]
- 12.Denning D.W. Invasive aspergillosis. Clin. Infect. Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 13.El-Shanawany A.A., Mostafa M.E., Barakat A. Fungal populations and mycotoxins in silage in Assiut and Sohag governorates in Egypt, with a special reference to characteristic Aspergilli toxins. Mycopathologia. 2005;159:281–289. doi: 10.1007/s11046-004-5494-1. [DOI] [PubMed] [Google Scholar]
- 14.Frisvad J.C., Rank C., Nielsen K.F., Larsen T.O. Metabolomics of Aspergillus fumigatus. Med. Mycol. 2009;47:S53–S71. doi: 10.1080/13693780802307720. [DOI] [PubMed] [Google Scholar]
- 15.Niimi K., Harding D.R., Parshot R., King A., Lun D.J., Decottignies A., Niimi M., Lin S., Cannon R.D., Goffeau A., et al. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a d-octapeptide derivative. Antimicrob. Agents Chemother. 2004;48:1256–1271. doi: 10.1128/AAC.48.4.1256-1271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavigne J.P., Brunel J.M., Chevalier J., Pages J.M. Squalamine, an original chemosensitizer to combat antibiotic-resistant gram-negative bacteria. J. Antimicrob. Chemother. 2010;65:799–801. doi: 10.1093/jac/dkq031. [DOI] [PubMed] [Google Scholar]
- 17.Xue T., Nguyen C.K., Romans A., May G.S. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot. Cell. 2004;3:557–560. doi: 10.1128/EC.3.2.557-560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes G., Romans A., Nguyen C.K., May G.S. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot. Cell. 2006;5:1934–1940. doi: 10.1128/EC.00178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saccharomyces Genome Database. [accessed on 5 September 2012]. Available online: http://www.yeastgenome.org.
- 20.Clinical and Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard. Second Edition. Vol. 22 CLSI; Wayne, PA, USA: 2008. [Google Scholar]
- 21.Odds F. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 22.Statistics to Use. [accessed on 5 September 2012]. Available online: http://www.physics.csbsju.edu/stats/
- 23.Clemons K.V., Schwartz J.A., Stevens D.A. Therapeutic and toxicologic studies in a murine model of invasive pulmonary aspergillosis. Med. Mycol. 2011;49:834–847. doi: 10.3109/13693786.2011.577822. [DOI] [PubMed] [Google Scholar]
- 24.Sokol-Anderson M.L., Brajtburg J., Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 1986;154:76–83. doi: 10.1093/infdis/154.1.76. [DOI] [PubMed] [Google Scholar]
- 25.Graybill J.R., Burgess D.S., Hardin T.C. Key issues concerning fungistatic versus fungicidal drugs. Eur. J. Clin. Microbiol. Infect. Dis. 1997;16:42–50. doi: 10.1007/BF01575120. [DOI] [PubMed] [Google Scholar]
- 26.An M., Shen H., Cao Y., Zhang J., Cai Y., Wang R., Jiang Y. Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans. Int. J. Antimicrob. Agents. 2009;33:258–263. doi: 10.1016/j.ijantimicag.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 27.González-Párraga P., Sánchez-Fresneda R., Zaragoza O., Argüelles J.C. Amphotericin B induces trehalose synthesis and simultaneously activates an antioxidant enzymatic response in Candida albicans. Biochim. Biophys. Acta. 2011;1810:777–783. doi: 10.1016/j.bbagen.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto Y., Aoki S., Mataga I. Enhancement of amphotericin B activity against Candida albicans by superoxide radical. Mycopathologia. 2004;158:9–15. doi: 10.1023/b:myco.0000038430.20669.80. [DOI] [PubMed] [Google Scholar]
- 29.Arendrup M.C., Cuenca-Estrella M., Lass-Flörl C., Hope W. the EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2. Clin. Microbiol. Infect. 2012;18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 30.Heinisch J.J., Lorberg A., Schmitz H.P., Jacoby J.J. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 1999;32:671–680. doi: 10.1046/j.1365-2958.1999.01375.x. [DOI] [PubMed] [Google Scholar]
- 31.Hahn J.S., Thiele D.J. Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J. Biol. Chem. 2002;277:21278–21284. doi: 10.1074/jbc.M202557200. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.H., Campbell B.C., Mahoney N., Chan K.L., May G.S. Targeting antioxidative signal transduction and stress response system: Control of pathogenic Aspergillus with phenolics that inhibit mitochondrial function. J. Appl. Microbiol. 2006;101:181–189. doi: 10.1111/j.1365-2672.2006.02882.x. [DOI] [PubMed] [Google Scholar]
- 33.Bartlett D.W., Clough J.M., Godwin J.R., Hall A.A., Hamer M., Parr-Dobrzanski B. The strobilurin fungicides. Pest Manag. Sci. 2002;58:649–662. doi: 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- 34.Kojima K., Takano Y., Yoshimi A., Tanaka C., Kikuchi T., Okuno T. Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 2004;53:1785–1796. doi: 10.1111/j.1365-2958.2004.04244.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim J.H., Mahoney N., Chan K.L., Molyneux R.J., May G.S., Campbell B.C. Chemosensitization of fungal pathogens to antimicrobial agents using benzo analogs. FEMS Microbiol. Lett. 2008;281:64–72. doi: 10.1111/j.1574-6968.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 36.Becker W.F., von Jagow G., Anke T., Steglich W. Oudemansin, strobilurin A, strobilurin B, and myxothiazol: new inhibitors of the bc1 segment of the respiratory chain with an E-β-methoxyacrylate system as common structural element. FEBS Lett. 1981;132:329–333. doi: 10.1016/0014-5793(81)81190-8. [DOI] [PubMed] [Google Scholar]
- 37.Takimoto H., Machida K., Ueki M., Tanaka T., Taniguchi M. UK-2A, B, C and D, novel antifungal antibiotics from Streptomyces sp. 517–02. IV. Comparative studies of UK-2A with antimycin A3 on cytotoxic activity and reactive oxygen species generation in LLC-PK1 cells. J. Antibiot. 1999;52:480–484. doi: 10.7164/antibiotics.52.480. [DOI] [PubMed] [Google Scholar]
- 38.Vincent J.M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 1947;159:850. doi: 10.1038/159850b0. [DOI] [PubMed] [Google Scholar]