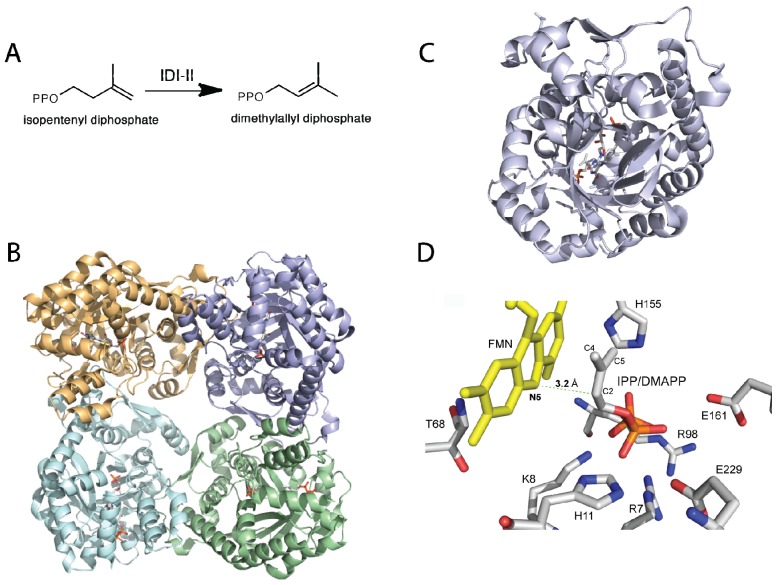
Figure 2.
(A) Reaction catalyzed by isopentenyl diphosphate/dimethylallyl diphosphate isomerase (IDI)-II; (B) Cartoon representation of IDI-II from Sulfolobus shibatae (PDB code, 2ZRY), showing each monomer in a different color; (C) Top view of a monomer of IDI-II showing the FMN bound at the center of the TIM barrel; (D) Residues in the active site predicted to play a role in substrate binding. The position of IPP in the active site shows that only the flavin N5 is in proper orientation to function as an acid/base during catalysis.