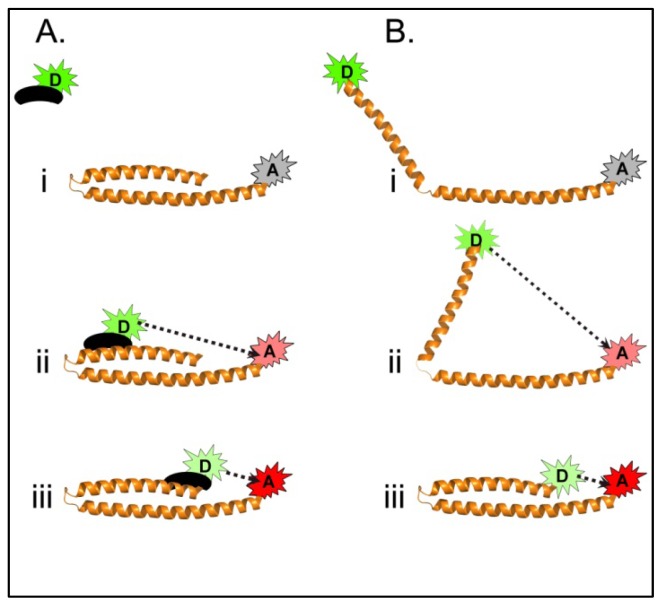
Figure 3.
Schematic of hypothetical FRET systems probing intermolecular (A) and intramolecular (B) energy transfer. (A) In this intermolecular example, an acceptor fluorophore is bound to a protein of interest and a donor fluorophore is attached to a ligand which could represent a small molecule, peptide, or another protein. When unbound (Ai), the ligand-associated donor is separated by too much distance to allow efficient energy transfer to occur. Upon binding of the ligand to the protein, the binding event can be monitored by an increase in energy transfer efficiency (seen by the dashed line in Aii and Aiii). Depending on the complexity of the protein structure, the measured energy transfer efficiencies and calculated distances between the probes can be used to help identify the relative location of the ligand binding site (Aii and Aiii). (B) Measurement of intramolecular FRET can be used to gain insight into protein conformations and conformational changes following a specific event such as a molecular interaction. In this example, a protein conformational change results in the intramolecular FRET pair moving from a distance too great for energy transfer to be reliably observed (Bi), to an intermediate distance with detectable, but minimal, energy transfer (Bii), to a final conformation resulting in the two probes being within close proximity to each other and which gives rise to a maximal observed energy transfer efficiency (Biii).