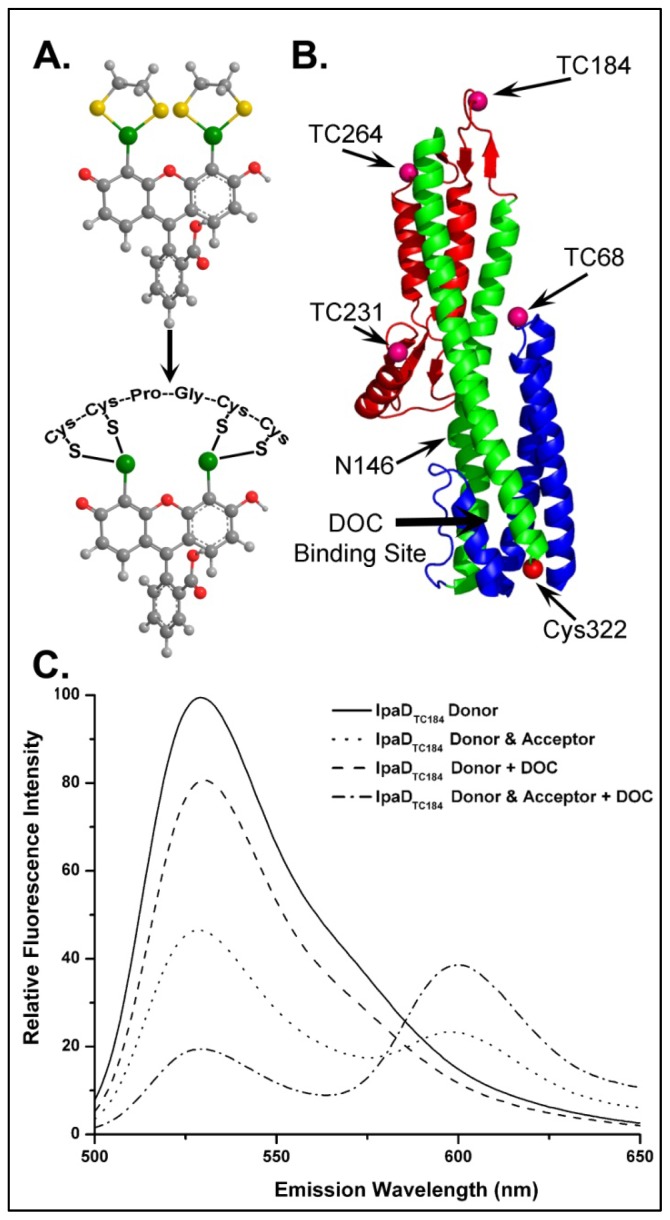
Figure 4.
Location of tetracysteine (TC) FlAsH binding sites engineered into IpaD and the fluorescence emission spectra used to quantify FRET from FlAsH on TC184 to Alexa 568 on Cys322. FlAsH is a fluorescein-based fluorophore that specifically coordinates to the TC sequence Cys-Cys-Pro-Gly-Cys-Cys, resulting in a dramatic increase in quantum yield [51] (A). The residues at which TC pockets were introduced into IpaD are indicated on the ribbon structure (B). Panel C shows the representative fluorescence spectra used to determine the effect of DOC on energy transfer efficiencies between FlAsH and the acceptor (Alexa 568) at the native Cys in the IpaD TC184 mutant. As described in the text, energy transfer efficiencies were quantified by measuring the change in donor fluorescence emission intensity in the presence and absence of an acceptor. The spectra in panel C not only show that the donor emission is decreased in the presence of the FRET acceptor, but that the extent of the change is dependent on DOC, allowing a systematic measure of the effect of DOC binding on IpaD structure. The key for identification of each spectrum is given in the inset. Figure adapted from [4].