Figure 3.
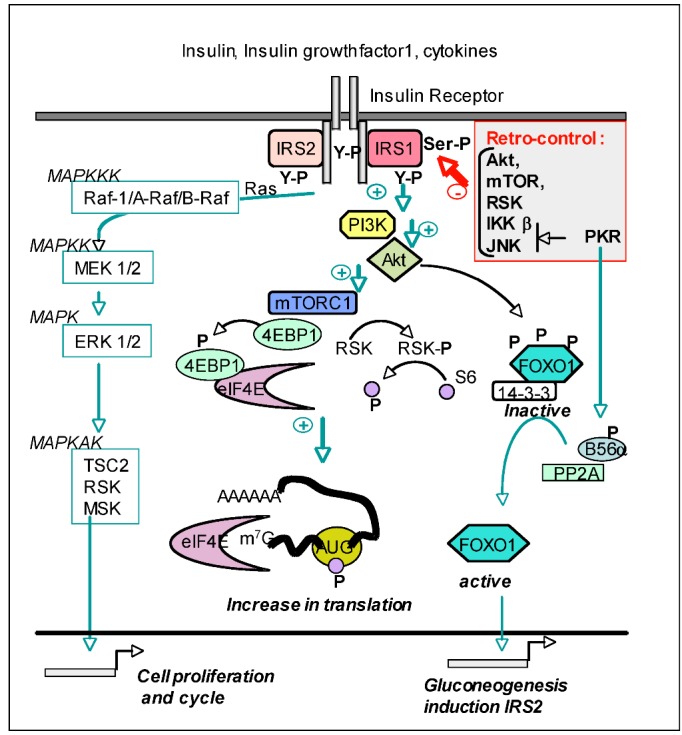
PKR and the Insulin signaling pathway. After binding of the insulin receptor to its different ligands, its intracytosolic chains autophosphorylate on tyrosine residues and phosphorylate the two substrates IRS1 and IRS2 on tyrosine residues to recruit the SH2‑containing Phosphoinositol-3 kinase (PI3K). Lipid products generated by PI3K bind and activate Akt, which in turn activates mTORC1. The latter phosphorylates 4EBP1 and RSK (Ribosomal S6 kinase). As a result, 4EBP1 dissociates from the cap-binding eIF4E protein and RSK phosphorylates the S6 ribosomal protein. The outcome of this is an increase in translation. Activation of the insulin receptor triggers also the Mitogen Activated Protein Kinase (MAPK) cascade (reviewed in [85]) leading to the transcription of factors required for cell proliferation and cycle. Akt also negatively regulates the Forkhead BoxO transcription factor FOXO1 by phosphorylation. This maintains FOXO1 in the cytosol in a complex with the 14-3-3 protein and prevents gluconeogenesis or transcription of IRS2 when not needed. Retro-control of the insulin pathway occurs through phosphorylation of IRS1 on serine residues by Akt, mTOR, RSK and by PKR, the latter acting through the intermediate IKKβ or JNK kinases. This prevents its interaction with the insulin receptor. However, PKR can also positively activate the insulin signaling by triggering FOXO1 dephosphorylation through the phosphorylation of B56α, the regulatory subunit of the protein phosphatase PP2A.
