Abstract
Background: Lysinuric protein intolerance (LPI) is an autosomal recessive cationic amino acid transport defect characterized by episodes of postprandial hyperammonemias and spontaneous protein aversion. Subnormal growth is common in spite of appropriate nutritional therapy. Growth hormone (GH) therapy promotes appetite, protein synthesis and accretion, but its possible growth-promoting effects and safety in patients with LPI are poorly known.
Methods: Four LPI children aged 7–16 years were treated with GH for a period of 3–4.5 years. Dietary intakes and plasma amino acid levels were analyzed frequently in addition to routine monitoring of GH therapy.
Results: Insulin-like growth factor-1 concentration was low and bone age was delayed in all LPI patients, but GH provocative test was pathological in only one of the patients. During the 3–4.5 years of GH therapy (dose 0.035–0.050 mg/kg/day), bone age did not catch up but height standard deviation score (SDS) improved by 0.7–1.8 SDS. There were no episodes of hyperammonemias.
Conclusions: Our data support safety and growth-promoting potential of long-term GH therapy in patients with LPI.
Keywords: Children, Growth, Growth hormone, Lysinuric protein intolerance, Nutrition
Introduction
Lysinuric protein intolerance (LPI) is an autosomal recessive cationic amino acid transport defect (Perheentupa and Visakorpi 1965), which is caused by mutations in SLC7A7 (Torrents et al. 1999). LPI is exceptionally common in Finland and almost half of the >100 reported patients are of Finnish origin (Simell 2001). The amino acid transporter y+LAT-1 regulates intestinal absorption and renal loss of cationic amino acids (Simell 2001). Defect in the transporter leads to low plasma concentrations of lysine, arginine, and ornithine and the ensuing paucity of the urea cycle intermediates may increase the risk of hyperammonemia.
Treatment of LPI aims at prevention of hyperammonemia and optimization protein nutrition by protein restriction and supplementation with oral l-citrulline and lysine-HCl and, occasionally, with sodium benzoate or sodium phenylbutyrate (Simell 2001). Although current treatment regime combined with nutritional therapy effectively prevents acute episodes of hyperammonemia, the patients are at risk for malnutrition due to severe protein aversion. Consequently, subnormal growth is common in children with LPI.
Growth hormone (GH) promotes protein synthesis and accretion while reducing protein breakdown (Copeland and Nair 1994). It also stimulates food and protein intake (Roberts et al. 1995; Veyrat-Durebex et al. 1999; Blissett et al. 2000). GH action is mediated through insulin-like growth factor-1 (IGF-1) and hepatic IGF-1 synthesis is compromised in malnutrition (Palacio et al. 2002). There is a report on GH therapy in patients with organic acidemias with and without GH deficiency concluding that GH may be of value in some metabolic diseases (Marsden et al. 1994). We thus hypothesized that LPI patients might also benefit from GH therapy, but use of GH in metabolic diseases may also carry risks due to increased food consumption and subsequent potential overload of amino acids or other nutrients. To date, there is only one report of short-term GH therapy in LPI in a 12-year-old severely stunted girl with renal tubular disease as well (Esposito et al. 2006). To strengthen the proof of concept, we have treated four LPI children aged 7–16 years with recombinant GH for several years. We now report here the effects of GH therapy on growth and protein nutrition in these children.
Methods
Patient characteristics are presented in Table 1. All patients are Finnish and homozygous for the Finnish LPI founder mutation LPI(Fin) 1181-2A → T. Three of the patients had renal disease with poor growth, which is by itself an indication for GH therapy (Haffner et al. 2000). One patient was started on GH empirically to improve her growth.
Table 1.
Patient characteristics during GH therapy
| Patient no. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | F | F | F | M |
| Age (year) | 10.7 | 12.4 | 15.8 | 16.5 |
| Medications | ||||
| Citrulline (mg/kg/day) | 120 | 133 | 104 | 53 |
| Lysine (mg/kg/day) | 16 | 26 | 7 | 15 |
| Leucine (mg/day) | – | – | 1,000 | – |
| Isoleucine (mg/day) | – | – | 500 | – |
| Sodium benzoate (mg/kg/day) | 97 | – | 108 | 81 |
| Sodium phenylbutyrate (mg/kg/day) | 182 | 112 | 149 | 168 |
| l-carnitine (g/day) | 1 | 1 | 1 | 1 |
| Calcium (mg/day) | 500 | 1,500 | 500 | 1,000 |
| Phosphate (mg/day) | – | 1,000 | – | – |
| Multivitamin (tabl/day) | 1 | 1 | 1 | 1 |
| Essential amino acid mixture (g/day) | 15 | – | – | 12 |
| Glucose polymer/corn starch (g/day) | 100 | 25 | 100 | – |
| Na-phosphate solution (Joulie, ml/day) | – | – | 36 | 33 |
| Citrate solution (Lightwood-I, ml/day) | – | – | 150 | – |
| Na hydrogenocarbonate (g/day) | – | 2.5 | – | 9 |
| Lovastatin (mg/day) | – | – | 10 | – |
| Other | – | – | Leuprorelin | – |
| Laboratory | ||||
| Creatinine (μmol/l) | 34 | 79 | 131 | 95 |
| Cystatine C (mg/l) | 0.71 | 1.54 | 1.53 | 1.39 |
| Creatinine clearance (ml/s/1.73 m2) | – | 0.46 | 0.65 | – |
| HCO3 | – | 20 | 20 | 18 |
| Total cholesterol (mmol/l) | 4.6 | 5.3 | 4.0 | 5.2 |
| HDL cholesterol (mmol/l) | 0.79 | 1.08 | 1.03 | 1.05 |
| Triglycerides (mmol/l) | 3.4 | 5.7 | 4.2 | 3.4 |
Heights (to the nearest 0.1 cm) and weights (to the nearest 0.1 kg) were measured using Harpenden stadiometer and Soehnle S10 electronic scale, respectively. Yearly blood samples (for measurement of CBC, free T4, HbA1c, IGF-1, NH4, prealbumin, serum total and HDL-cholesterol and triglycerides, creatinine, cystatin-C, as well as plasma amino acids) were drawn after fasting, and the analyses were performed at the Central Laboratory of the Turku University Hospital using standardized methods. Insulin–arginine and clonidine provocation tests were performed using standard protocols. Bone age (Bayley–Pinneau) was estimated from the left hand and wrist. Estimation of dietary intakes was based on food records, which were checked for accuracy by a nutritionist.
Results
IGF-1 Values, GH Provocative Tests and Effect of GH Therapy on Growth
IGF-1 values were low in all LPI patients before GH therapy (Table 2) but increased as expected during treatment. GH stimulation test was abnormal only in one of the patients and bone age was significantly delayed in all patients. Daily GH doses between 0.035 mg/kg and 0.050 mg/kg/day were used. Two of the patients (patients 1 and 2, both female) were prepubertal at the initiation of the GH therapy and remained so for years on GH therapy. Patient 3 (female) had entered puberty at the initiation of GH therapy, and she was treated with GnRH-analog (leuprorelin 75 mg/kg every 4 weeks) simultaneously with GH. Patient 4 (male) was just entering puberty (G2P1) at the initiation of the GH therapy.
Table 2.
The effect of GH treatment in LPI patients
| Patient no. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | F | F | F | M |
| Age at introduction of GH therapy (year) | 7.2 | 7.9 | 11.8 | 13.5 |
| GH dose (mg/kg/day) | 0.050 | 0.035 | 0.050 | 0.035 |
| GH duration (year) | 3.5 (ongoing) | 4.5 (ongoing) | 4 (ongoing) | 3 (ongoing) |
| Antropometry | – | – | – | – |
| Birth weight (kg) | 3.270 | 2.980 | 4.020 | 3.740 |
| Birth length (cm) | 50.5 | 48 | 52 | 52 |
| Height (cm) at GH introduction | 105.2 | 97.4 | 129.2 | 134.4 |
| Height (SD) at GH introduction | −3.4 | −5.5 | −2.9 | −2.7 |
| Weight (kg) at GH introduction | 15.8 | 14.4 | 26.9 | 29.7 |
| Weight (%)a at GH introduction | −8 | −5 | +1 | +1 |
| Growth velocity 1 year prior to GH (cm/year) | 4.8 | 4.0 | 7.4 | 4.2 |
| Growth velocity during 1st year on GH (cm/year) | 10.0 | 7.8 | 6.6 | 9.6 |
| Current height (cm) | 126.1 | 126.7 | 151.2 | 159.7 |
| Current height (SD) | −2.1 | −3.7 | −2.1 | −2.0 |
| Laboratory | – | – | – | – |
| GH max (insulin-arginine-test, μg/l) | 12.6 | 8.4 | na | 11.7 |
| GH max (clonidine test, μg/l) | na | na | na | 6.5 |
| IGF-1 before GH treatment (nmol/l) | 8 | <5 | 11 | 13 |
| IGF-1 during GH treatment (nmol/l) | 10 | 11.6 | 40 | 49 |
| Plasma arginineb before GH (μmol/l) | 25 | 34 | 37 | 23 |
| Plasma arginine during GH (μmol/l) | 14 | 43 | 41 | 30 |
| Plasma lysinec before GH (μmol/l) | 82 | 74 | 144 | 89 |
| Plasma lysine during GH (μmol/l) | 65 | 120 | 141 | 71 |
| Plasma glutamined before GH (μmol/l) | 2,181 | 1,682 | 1,331 | 963 |
| Plasma glutamine during GH (μmol/l) | 1,353 | 1,071 | 952 | 1,287 |
| Urinary orotate before GH (μmol/mmol crea) | 9.7 | 8.3 | 3.9 | 5.0 |
| Urinary orotate during GH (μmol/mmol crea) | 4.2 | 6.9 | 3.3 | 4.0 |
| Plasma prealbumin before GH (mg/l) | 166 | na | 207 | 270 |
| Plasma prealbumin during GH (mg/l) | 185 | na | 191 | 180 |
| Bone agee | – | – | – | – |
| Before GH | −2 year | −3 year | −2.5 year | −4.5 year |
| After 2–3 years on GH | −2 year | −2.5 year | −2 year | −2.5 year |
| Nutrition and amino acid doses | – | – | – | – |
| Protein intake before GH (g/kg/day) | 1.1 | 1.3 | 1.2 | 1.4 |
| Protein intake during GH (g/kg/day) | 1.1 | 1.2 | 1.2 | na |
| Citrulline dose before GH (mg/kg/day) | 132 | 182 | 130 | 99 |
| Citrulline dose during GH (mg/kg/day) | 120 | 133 | 104 | 53 |
| Lysine dose before GH (mg/kg/day) | 20 | 29 | 9 | 17 |
| Lysine dose before GH (mg/kg/day) | 16 | 26 | 7 | 15 |
aPercentual deviation from age and gender adjusted mean weight
bReference range 23–86 μmol/l (from Dickenson et al. 1965)
cReference range 71–151 μmol/l
dReference range 57–467 μmol/l
eBone age – calendar age
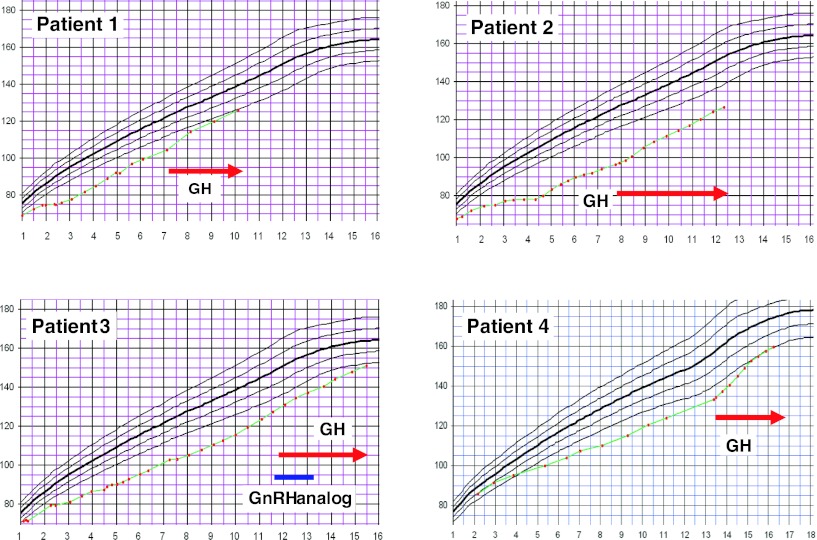
During the 3–4.5 years of GH therapy, height standard deviation score (SDS) improved by 0.7–1.8 SDS (Fig. 1). First-year growth velocity increased in all but one patient (Patient 3), who was treated with GnRH-analog (see above). Bone age did not catch up during GH therapy, but IGF-1 values increased as expected (Table 2).
Fig. 1.
Growth curves of four LPI patients with growth hormone therapy. GH growth hormone, GnRH analog gonadotropin-releasing hormone analog
Nutrition and Dietary Intakes
Protein intake, in gram per kilogram per day, showed no clear differences during the GH therapy, although it increased when expressed in grams/day (Table 2). Oral citrulline and lysine doses showed a slight decreasing trend. Plasma arginine and lysine as well as prealbumin concentrations remained rather stable but plasma glutamine decreased markedly in three of the patients during GH therapy. There were no episodes of hyperammonemia or other adverse events. GH treatment was therefore well tolerated by all four LPI patients.
Discussion
Long-term recombinant GH therapy was effective and well tolerated in four LPI children aged 7–16 years of age as judged by improved growth and protein tolerance. The GH therapy was also safe as it was continued for more than 3 years without any apparent adverse events. Two of the female patients were prepubertal through many years on GH therapy but one female was pubertal at the GH onset. One male subject entered puberty at the start of GH treatment but, as the growth acceleration takes place in Tanner stage 3–4 in boys, his improved growth velocity was not due to pubertal growth spurt. Taken together, GH therapy offers new possibilities to improve growth in LPI. However, the effect of GH on adult height achievement could not be measured as the patients have not yet reached adult height.
In LPI, spontaneous aversion to dietary protein develops at an early age. The LPI patients are encouraged to increase dietary protein intake modestly during citrulline and nitrogen-scavenging therapy, but aversion prevents many patients from accepting more than minimal requirements (Simell 2001). Children with LPI often show retarded growth and poor muscular development. Inadequate nutrition due to protein aversion may well contribute to the poor growth in LPI (Simell 2001) and some of the pathological manifestations of LPI may be related to deficiency of diamino acids (lysine, arginine, ornithine) and other essential amino acids, but the pathogenesis of the many problems associated with this disease is still poorly understood (Rajantie et al. 1980; Sidransky and Verney 1985; Simell 2001; Tanner et al. 2007). Compromised growth is not alleviated with citrulline supplementation. It may therefore be more related to reduced lysine availability than defective urea cycle function in LPI (Awrich et al. 1975; Tanner et al. 2007).
Interestingly, a recent study reported severe intrauterine growth restriction in LPI (Slc7a7-deficient) mice with markedly downregulated IGF-1 expression in fetal liver. Moreover, only a minority of LPI pups survived indicating clearly more severe phenotype in LPI mice than in humans (Sperandeo et al. 2007). In humans with LPI, intrauterine growth appears to be normal and growth failure is a postnatal phenomenon. The infants thrive as long as they are breast-fed (Simell et al. 1975), whereas in mice the growth is retarded already in utero (Bröer 2007). In keeping with this, the LPI children in our study did not express intrauterine growth restriction, since their birth lengths varied between 48 and 52 cm and weights between 2,980 and 4,020 g.
Patients with LPI have decreased plasma levels of arginine, ornithine, and lysine. Intravenous arginine inhibits somatostatin release, and it is commonly used as GH-releasing stimulant in GH secretion tests. Oral arginine, when given in large doses, stimulates GH secretion (Collier et al. 2005), and oral arginine supplementation has been associated with improved growth in two children with LPI (Goto et al. 1984). Oral lysine ingestion acutely and markedly increases serum GH concentrations as well (van Vught et al. 2008). We use oral lysine supplementation in moderate doses to normalize plasma lysine values and oral citrulline to boost urea cycle. Oral arginine and large doses of oral lysine are not tolerated well in LPI since they may cause diarrhea and other abdominal complaints. Still, growth of many LPI patients remains compromised.
It is well known that GH promotes protein synthesis, protein accretion, and lipolysis and reduces protein breakdown (Copeland and Nair 1994). GH also stimulates food and protein intake (Roberts et al. 1995; Veyrat-Durebex et al. 1999; Blissett et al. 2000). GH/IGF axis is influenced by many factors, including malnutrition and renal disease, as recently reviewed by Richmond and Rogol (2008). In those situations, IGF-values are decreased whereas GH values are increased due to GH resistance (Grottoli et al. 2003). Many patients with inherited metabolic disease show impaired growth, but only few have been successfully alleviated with GH. It has, however, been used in patients with organic acidemias with and without GH deficiency but the value of it has remained unsure (Marsden et al. 1994). Prader–Willi children show increased anabolism and lean body mass and less fat mass gain if treated with GH (Eiholzer and Whitman 2004). Children with chronic renal failure show catch-up growth when given GH treatment and majority of them achieve normal adult height (Haffner et al. 2000). Previously, there is only one report of short-term GH therapy in a 12-year-old severely growth-retarded girl with LPI and tubular disease (Esposito et al. 2006). She had GH deficiency (peak GH secretion in a stimulation test was 6.4 μg/l) and presented a significant acceleration of growth from 2 cm/year before therapy to 8 cm/year during the first year on GH. Our four LPI patients showed analogous improvement of growth. Possible side effects of GH include slipped femoral epiphysis and benign cerebral hypertension; both of these might be more probable in LPI patients, who are prone to hyperammonemia-induced brain dysfunction and osteoporosis. However, neither of these complications was observed in our patients and the GH therapy was also otherwise well tolerated. In fact, decreased plasma glutamine concentrations may indicate a reduced risk of clinical hyperammonemia.
The weakness of this study is the low number of subjects and any decisive conclusions cannot be drawn. Moreover, two of the patients (one boy and one girl) were pubertal at the start of GH therapy. However, in boys the growth acceleration typically does not take place before Tanner stage 3, and the girl received also GnRH-therapy to delay the advance in bone age development.
In conclusion, patients with LPI show retarded growth, but GH therapy alleviated growth failure in 4 LPI children without measurable adverse effects on protein metabolism.
Acknowledgments
This study was supported by the European Genomics Initiative on Disorders of Plasma Membrane Amino Acid Transporters (EUGINDAT), the Finnish Cultural Foundation, and the Novo Nordisk Foundation.
Abbreviations
- GH
Growth hormone
- IGF-1
Insulin-like growth factor-1
- LPI
Lysinuric protein intolerance
- SLC7A7
Solute carrier family 7, member 7
Take Home Message
Poor growth in children with lysinuric protein intolerance can be improved by growth hormone.
References
- Awrich AE, Stackhouse WJ, Cantrell JE, Patterson JH, Rudman D. Hyperdibasicaminoaciduria, hyperammonemia, and growth retardation: treatment with arginine, lysine and citrulline. J Pediatr. 1975;87:731–738. doi: 10.1016/S0022-3476(75)80296-4. [DOI] [PubMed] [Google Scholar]
- Blissett J, Harris G, Kirk J. Effect of growth hormone therapy on feeding problems and food intake in children with growth disorders. Acta Paediatr. 2000;89:644–949. doi: 10.1111/j.1651-2227.2000.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Bröer S. Lysinuric protein intolerance: one gene, many problems. Am J Physiol Cell Physiol. 2007;293:C540–C541. doi: 10.1152/ajpcell.00166.2007. [DOI] [PubMed] [Google Scholar]
- Collier SR, Casey DP, Kanaley JA. Growth hormone responses to varying doses of oral arginine. Growth Horm IGF Res. 2005;15:136–139. doi: 10.1016/j.ghir.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Nair KS. Acute growth hormone effects on amino acid and lipid metabolism. J Clin Endocrinol Metab. 1994;78:1040–1047. doi: 10.1210/jc.78.5.1040. [DOI] [PubMed] [Google Scholar]
- Dickenson JC, Rosenblum H, Hamilton PB. Ion exchange chromatography of the free amino acids in the plasma of the newborn infant. Pediatrics. 1965;36:2–13. [PubMed] [Google Scholar]
- Eiholzer U, Whitman BY. A comprehensive team approach to the management of patients with Prader-Willi syndrome. J Pediatr Endocrinol Metab. 2004;17:1153–1175. doi: 10.1515/JPEM.2004.17.9.1153. [DOI] [PubMed] [Google Scholar]
- Esposito V, Lettiero T, Fecarotta S, Sebastio G, Parenti G, Salerno M. Growth hormone deficiency in a patient with lysinuric protein intolerance. Eur J Pediatr. 2006;165:763–766. doi: 10.1007/s00431-006-0170-8. [DOI] [PubMed] [Google Scholar]
- Goto I, Yoshimura T, Kuroiwa Y. Growth hormone studies in lysinuric protein intolerance. Eur J Pediatr. 1984;141:240–242. doi: 10.1007/BF00572769. [DOI] [PubMed] [Google Scholar]
- Grottoli S, Gasco V, Ragazzoni F, Ghigo E. Hormonal diagnosis of GH hypersecretory states. J Endocrinol Invest. 2003;26(10 suppl):27–35. [PubMed] [Google Scholar]
- Haffner D, Schaefer F, Nissel R, Wuhl E, Tönshoff B, Mehls O. Effect of growth hormone treatment on the adult height of children with chronic renal failure. German study group for growth hormone treatment in chronic renal failure. N Engl J Med. 2000;343:923–930. doi: 10.1056/NEJM200009283431304. [DOI] [PubMed] [Google Scholar]
- Marsden D, Barshop BA, Capistrano-Estrada S, et al. Anabolic effects of human growth hormone: management of inherited disorders of catabolic pathways. Biochem Med Metab Biol. 1994;52:145–154. doi: 10.1006/bmmb.1994.1047. [DOI] [PubMed] [Google Scholar]
- Palacio AC, Pérez-Bravo F, Santos JL, Schlesinger L, Monckeberg F. Leptin levels and IgF-binding proteins in malnourished children: effect of weight gain. Nutrition. 2002;18:17–19. doi: 10.1016/S0899-9007(01)00690-6. [DOI] [PubMed] [Google Scholar]
- Perheentupa J, Visakorpi J. Protein intolerance with deficient transport of basic amino acids. Lancet. 1965;2:813–816. doi: 10.1016/S0140-6736(65)92446-3. [DOI] [PubMed] [Google Scholar]
- Rajantie J, Simell O, Perheentupa J. Basolateral-membrane transport defect for lysine in lysinuric protein intolerance. Lancet. 1980;1:1219–1221. doi: 10.1016/S0140-6736(80)91679-7. [DOI] [PubMed] [Google Scholar]
- Richmond EJ, Rogol AD. Growth hormone deficiency in children. Pituitary. 2008;11:115–120. doi: 10.1007/s11102-008-0105-7. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Azain MJ, White BD, Martin RJ. Rats treated with somatotropin select diets higher in protein. J Nutr. 1995;125:2669–2678. doi: 10.1093/jn/125.10.2669. [DOI] [PubMed] [Google Scholar]
- Sidransky H, Verney E. Chemical pathology of diamino acid deficiency: considerations in relation to lysinuric protein intolerance. J Exp Pathol. 1985;2:47–57. [PubMed] [Google Scholar]
- Simell O (2001) Lysinuric protein intolerance and other cationic aminoacidurias. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) Childs B, Kinzler KW, Vogelstein B (assoc. eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 4933–4956
- Simell O, Perheentupa J, Rapola J, Visakorpi JK, Eskelin LE. Lysinuric protein intolerance. Am J Med. 1975;59:229–240. doi: 10.1016/0002-9343(75)90358-7. [DOI] [PubMed] [Google Scholar]
- Sperandeo MP, Annunziata P, Bozzato A, et al. Slc7a7 disruption causes fetal growth retardation by downregulating Igf1 in the mouse model of lysinuric protein intolerance. Am J Physiol Cell Physiol. 2007;293:C191–C198. doi: 10.1152/ajpcell.00583.2006. [DOI] [PubMed] [Google Scholar]
- Tanner LM, Näntö-Salonen K, Niinikoski H, Huoponen K, Simell O. Long-term oral lysine supplementation in lysinuric protein intolerance. Metabolism. 2007;56:185–189. doi: 10.1016/j.metabol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Torrents D, Mykkänen J, Pineda M, et al. Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nat Genet. 1999;21:293–296. doi: 10.1038/6809. [DOI] [PubMed] [Google Scholar]
- van Vught AJAH, Nieuwenhuizen AG, Brummer R-JM, Westerterp-Plantenga MS. Effects of oral ingestion of amino acids and proteins on the somatotrophic axis. J Clin Endocrinol Metab. 2008;93:584–590. doi: 10.1210/jc.2007-1784. [DOI] [PubMed] [Google Scholar]
- Veyrat-Durebex C, Gaudreau P, Coxam V, Gaumet N, Alliot J. Peripheral injection of growth hormone stimulates protein intake in aged male and female rats. Am J Physiol. 1999;276:E1105–E1111. doi: 10.1152/ajpendo.1999.276.6.E1105. [DOI] [PubMed] [Google Scholar]