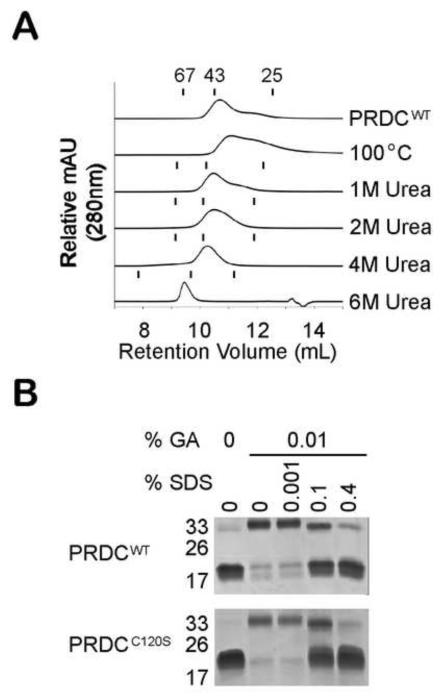
Figure 5. PRDCWT is a highly stable dimer.
A) Comparison of the elution profiles from SEC analysis of 100 μg of PRDCWT protein subjected to various denaturing conditions. For thermal analysis, PRDCWT was heated to 100°C and then analyzed at 25°C. For chemical denaturant analysis, PRDCWT was mixed with urea to a final concentration of 1 M, 2 M, 4 M or 6 M prior to loading onto a Superdex 75 column equilibrated under the same urea concentrations. The retention volume of the three MW standards (67, 43 and 25 kDa) under different urea concentrations are represented by tick marks above the corresponding elution profiles. B) SDS-PAGE analysis of PRDCWT and PRDCC120S cross-linked with 0.01% glutaraldehyde (GA) in the presence of increasing concentrations of SDS.