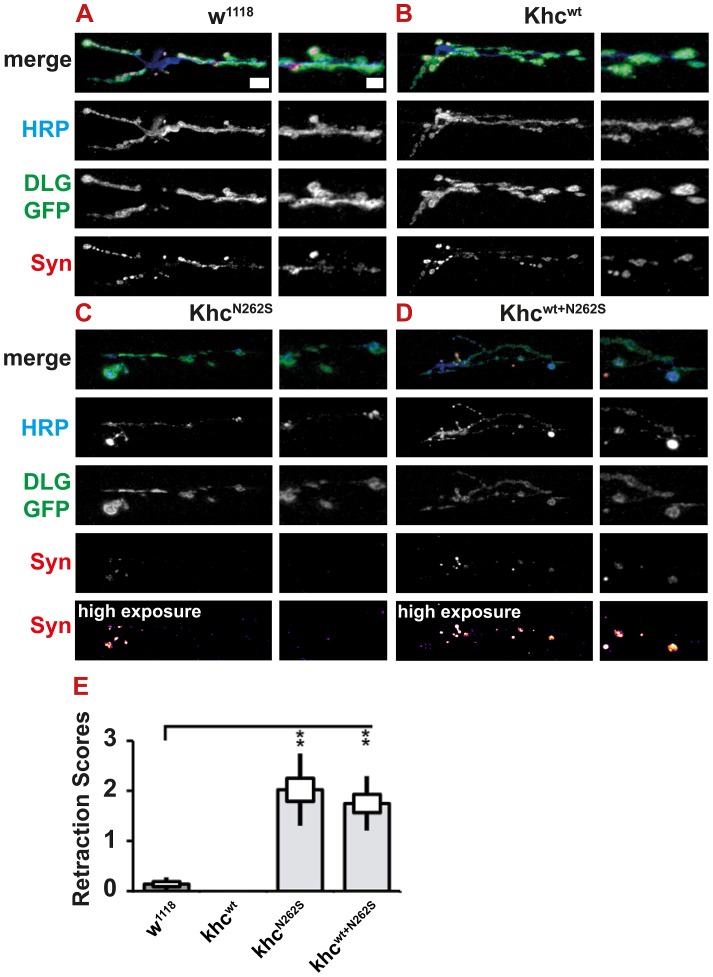
Figure 11. Light microscopic analysis of NMJ degeneration.
Degeneration was revealed and scored by immunofluorescent staining. All larvae carried one copy of the motoneuron-specific driver D42-Gal4 and were raised at 29°C. (A–D) Confocal images of immunofluorescent staining showing NMJs 6/7, segment A5 of mid-third-instar Drosophila larvae. To visualize the subsynaptic reticulum, we used a GFP insertion in the discs-large locus. Neuronal membranes were visualized with an antibody against horseradish peroxidase (HRP). Synaptic vesicles were stained using m-α-Synapsin (Syn) antibody. For KhcN262S- and Khcwt+N262S-expressing larvae, which showed a strong reduction in Syn intensity, an additional, false-colored panel (high exposure) is shown. In this panel, the brightness was adjusted for better visibility of weak signals. Scale bar: 10 µm; right panels 5 µm. Genotypes: (A) D42>w1118; (B) D42>Khcwt; (C) D42>KhcN262S; (D) D42>Khcwt+N262S. (E) To integrate the frequency of retractions, we used a neurodegenerative scoring system to combine the occurrence of dystrophic boutons and minor pathological alterations at the NMJs into a single measure for the degree of pathological alterations (for details see Figure S1A–S1F). Using this scoring system, we detected a significant degree of neurodegenerative alterations in larvae expressing KhcN262S either alone or in combination with Khcwt. Statistical significance was determined using a Kruskal-Wallis H-test followed by a Dunn's test for comparisons between multiple groups. The standard error of the mean (s.e.m.) is shown as a box, the standard deviation (s.d.) as a black line. ** p<0.01.