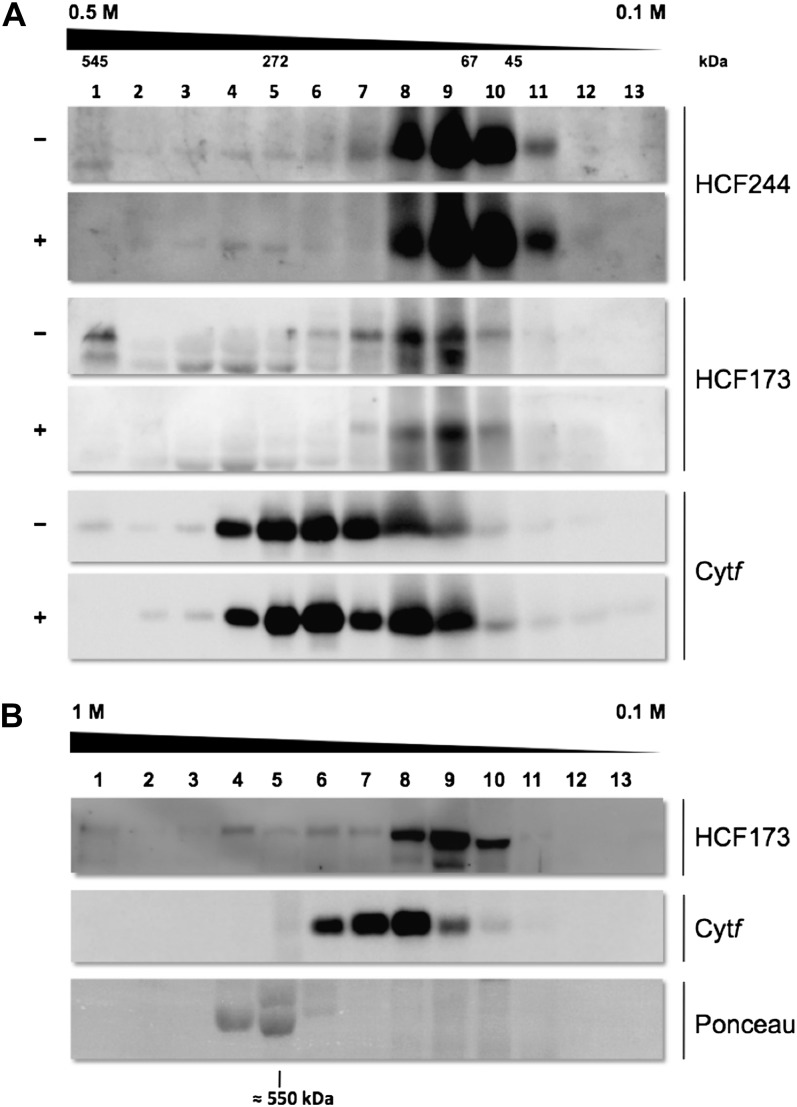
Figure 9.
Analysis of HCF complexes by discontinuous Suc gradient centrifugation. A, RNA association study. Wild-type thylakoid membrane proteins were solubilized with 1% (w/v) n-dodecyl-β-d-maltoside and treated with (+) or without (−) RNase A. An equivalent of 500 μg of chlorophyll was loaded on each gradient (0.5–0.1 m Suc). After centrifugation, 13 fractions were harvested. Proteins were precipitated with TCA and analyzed by SDS-PAGE. Exposure of immunoblots incubated with HCF244 antibody was increased to detect minor protein levels. B, Analysis of HCF173 complexes. Wild-type thylakoid membrane proteins were solubilized with 1% (w/v) digitonin and 0.5% n-dodecyl-β-d-maltoside. An equivalent of 500 μg of chlorophyll was loaded on the gradient (1–0.1 m Suc). The position of Rubisco is indicated by the Ponceau red staining of RbcL.