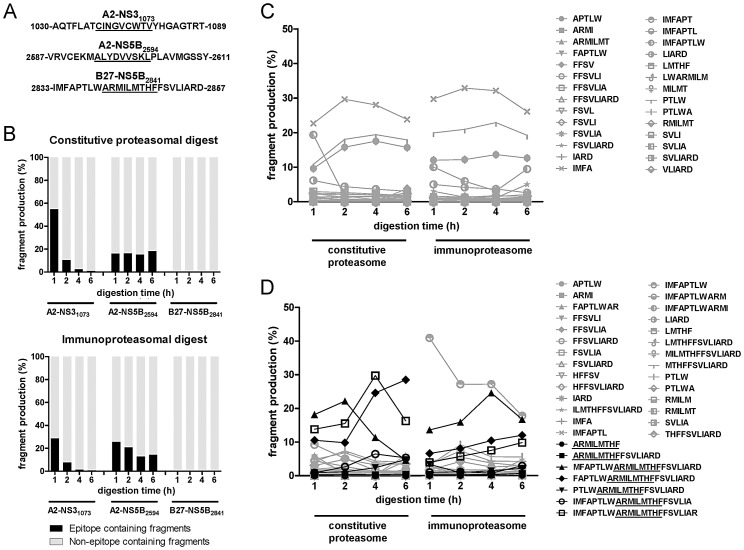
Figure 5. Proteasomal digestion of HCV-specific epitope-containing regions.
(A) Peptides of 24 (A2-NS31073) and 25 (A2-NS5B2594 and B27-NS5B2841) amino acids in length spanning the regions containing the respective epitopes were used for incubation with constitutive proteasomes and immunoproteasomes. Epitope sequences are underlined. (B) Production of epitope-containing and non-epitope-containing cleavage products of the 24-mer (A2-NS31073) and two 25-mer (A2-NS5B2594 and B27-NS5B2841) peptides incubated with constitutive proteasomes and immunoproteasomes for 1 to 6 hours using standard conditions. The sum of all fragment intensities was set at 100%. Data are representative of triplicate experiments. (C) The relative production of all cleavage products of the B27-NS5B2841 peptide by constitutive proteasomes and immunoproteasomes for 1 to 6 hours using standard conditions. All fragments were between 4 and 9 amino acids long. Data are representative of triplicate experiments. (D) The relative production of all cleavage products of the B27-NS5B2841 peptide by constitutive proteasomes and immunoproteasomes for 1 to 6 hours using a 4× dilution of both of the proteasomal forms. The abundance of B27-NS5B2841-containing fragments ending in a C-terminal 2857D represents a maximum as this corresponds to the end of the 25-mer peptide substrate. All fragments containing the NS5B2841 epitope are highlighted using black symbols. The NS5B2841 epitope sequence is underlined. Data are representative of triplicate experiments.