Abstract
Background
Airway obstruction and the extent of emphysema are reported to be responsible for reduced bone mineral density (BMD). Corresponding to different phenotypes of a pulmonary disease, different severity in extra pulmonary features may exist. We compared BMDs of subjects with or without airway obstruction and/or emphysema and investigated the relationships among BMD, the severity of airway obstruction, and the extent of emphysema.
Methods
Using a university hospital database, we reviewed patients over 40 years old who performed spirometry, computed tomography of chest, and measurement of BMD of the lumbar (L) spine. According to the presence or absence of airway obstruction and/or emphysema, four groups were classified.
Results
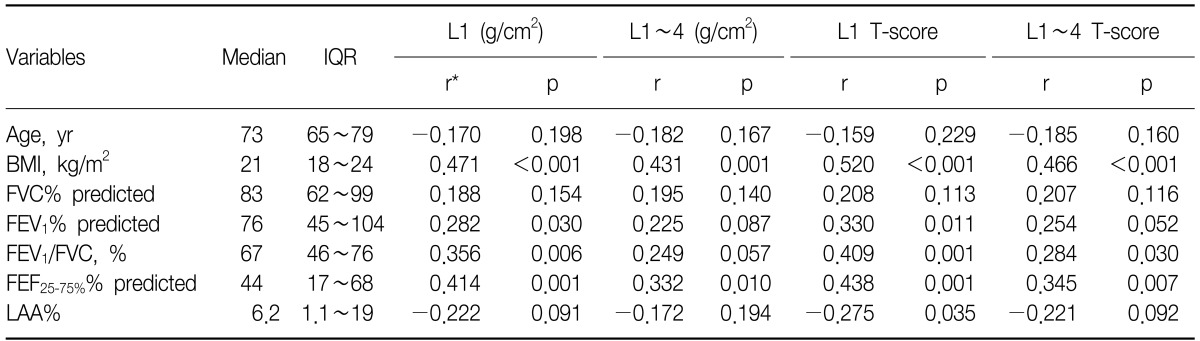
Among a total of 59 subjects, 33 (56%) had osteoporosis. The prevalence of osteoporosis in subjects with no airway obstruction and no emphysema, those with only emphysema, those with only airway obstruction, and those with both airway obstruction and emphysema were 42%, 57%, 64%, and 73%, respectively (p=0.047 by linear-by-linear association). The mean T-scores of BMD of L1 (p=0.032) and L1-4 spines were different among the four groups (p=0.034). Although the T-score of L1 BMD negatively correlated with the extent of emphysema (r=-0.275, p=0.035) and positively with each of body mass index (BMI) (r=0.520, p<0.001), forced expiratory volume in one second (FEV1) (r=0.330, p=0.011), FEV1/forced vital capacity (r=0.409, p=0.001), and forced expiratory flow at 25~75% of FVC (FEF25-75%) (r=0.438, p=0.0001), respectively, multiple linear regression analysis indicated that BMI (p<0.001) and FEF25-75% were predictive of BMD (p=0.012).
Conclusion
Low BMI and airway obstruction were strongly associated with reduced bone density rather than the extent of emphysema.
Keywords: Airway Obstruction, Bone Density, Pulmonary Emphysema, Osteoporosis, Body Mass Index
Introduction
Osteoporotic fractures decrease an individual quality of life and increase a socioeconomic burden. To predict the risk of osteoporotic fractures, measurements of bone mineral density (BMD) have been widely used. It has been reported that BMD is lower in patients with chronic obstructive pulmonary disease (COPD) than in healthy subjects1,2. COPD is currently understood to be associated with systemic inflammation as well as pulmonary inflammation3. Several factors such as the severity of airway obstruction and the extent of emphysema have been reported to be responsible for the reduced bone density in patients with COPD1,2,4-10. Recently, the extent of pulmonary emphysema was suggested to correlate with reduced bone density better than forced expiratory volume in one second (FEV1)9. However, there has been little study to investigate differences in BMDs or the prevalence of osteoporosis according to phenotypes of COPD, such as emphysema and airway obstruction, as well as control subjects. Our aims were to compare prevalence and BMDs among subjects in the presence or absence of airway obstruction and/or emphysema and investigate the relationships among BMD, the degree of airway obstruction, and the extent of emphysema.
Materials and Methods
1. Subjects
From medical record database of the Ewha Womans University Hospital from September 2003 through May 2009, we selected patients over 40 years who performed all three of spirometry, computed tomography (CT) of chest, and BMDs of lumbar spine within one-year period. Among them, we excluded those with significant lesions, such as interstitial lung disease, active pulmonary tuberculosis, tuberculous destroyed lung, pneumonia, lung cancer, and bronchiectasis, except emphysema, to affect lung volume measured by using CT images. Finally, 59 patients were retrospectively evaluated. The Institutional Review Board approved the analyses of the data.
2. Pulmonary function tests
Spirometry was performed as recommended by the American Thoracic Society using the Vmax 22 (Sensor Medics, Yorba Linda, CA, USA)11. The following values were evaluated: FEV1, forced vital capacity (FVC), the ratio of FEV1 to FVC (FEV1/FVC), forced expiratory flow at 25~75% of FVC (FEF25-75%). Airway obstruction is defined as a FEV1/FVC less than the lower limits of the normal range (LLN) for FEV1/FVC12,13.
3. Chest CT scans
Chest CT scans were performed on all patients at full inspiration using a 16-multi detector CT scanner (Somatom Sensation; Siemens Medical System, Forchheim, Germany). Images of the whole lung were extracted automatically and the attenuation coefficient of each pixel was calculated. The cutoff level between normal lung density and low-attenuation areas (LAA) was defined as -950 Hounsfield Units14. To evaluate pulmonary emphysema quantitatively, the total lung volume and the volume of LAA were automatically measured using a volume data set on a computer workstation (Rapidia 3D version 2.8; Infinitt Health Care, Seoul, Korea), and the percentage volume of LAA (%) was calculated as (volume of LAA)/(total lung volume)×100 (%). Emphysema is defined as having LAA% of more than 10%.
4. Measurements of BMD in vertebral bone
Bone mineral parameters were measured by using a Dual energy X-ray absorptiometry (Prodigy; GE-lunar, Houston, TX, USA) at the lumbar spine (vertebrae L1~4). Parameters were expressed in standard globally accepted terms: BMD (g/cm2). Standardized T-score analyses were used to compare individual bone density determinations for study subjects to those of a young normal control population of the same gender. This was done to standardize the BMD measurements to peak bone mass, which occurs at 30 years of age. The BMD measured is therefore correlated to the peak bone mass and is expressed as a T-score which is the number of standard deviations below or above peak bone mass for the relevant gender. T-score values between -1.0 and -2.5 are definable for osteopenia and T-scores below -2.5 are definable for osteoporosis15.
5. Statistical analysis
Statistical analysis was performed using SPSS-PC for Windows version 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive data are expressed as median value with interquartile range and frequencies are expressed as number (%). According to the presence or absence of airway obstruction and/or emphysema, four groups were classified. Differences between four groups were tested using the Kruskal-Wallis test. Prevalence of osteoporosis or osteopenia of four groups was compared by linear-by-linear association. To know relationship between a parameter of BMD and each variable, we used Pearson correlation analysis. To find out independent correlates of BMD, multiple linear regression analysis was used. Statistical significance was accepted at the p-values of <0.05 level.
Results
The mean age of our study subjects was 73 (interquartile range, 65~79) years and 33 patients (56%) were male. Among total 59 subjects, 47 had osteopenia or osteoporosis, and the prevalence of osteoporosis and osteopenia were 56% and 25%, respectively. Twenty-six patients (44%) had airway obstruction, and 22 (37%) had emphysema.
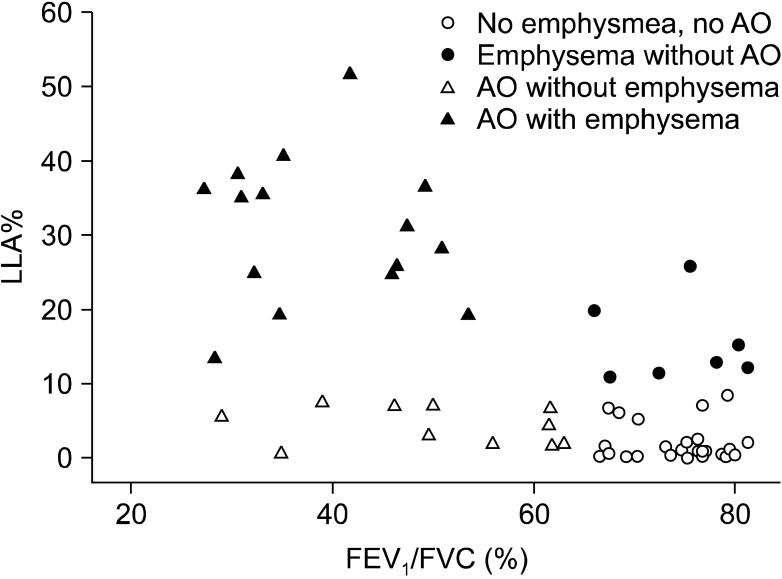
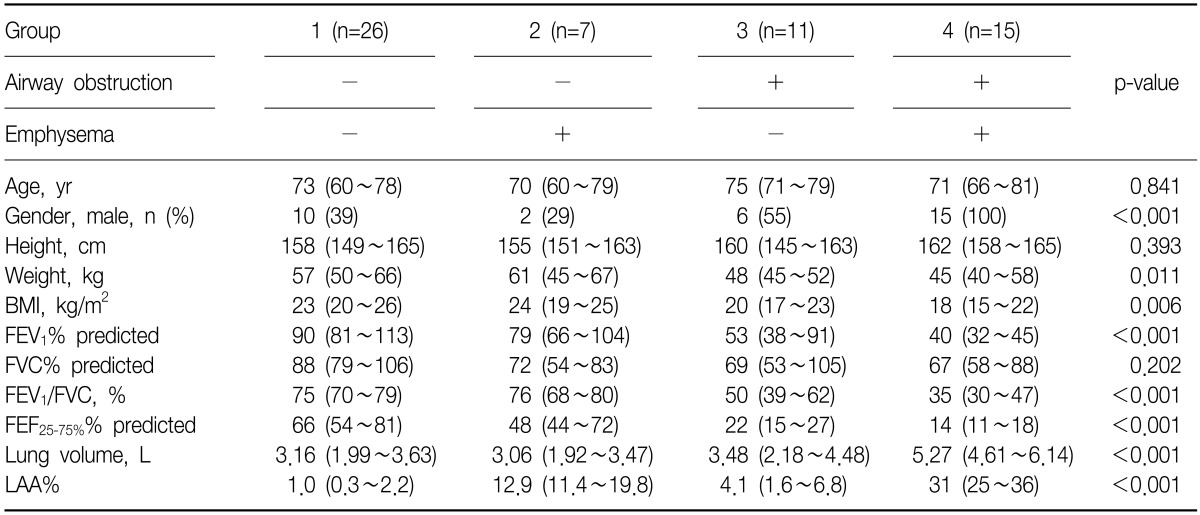
According to the presence or absence of airway obstruction and/or emphysema, four groups were classified (Figure 1). Group 1 consisting of 26 patients (44%) is characterized by no airway obstruction and no emphysema. Group 2 consisting of 7 patients (12%) is characterized by emphysema without airway obstruction. Group 3 includes 11 patients (19%) with airway obstruction without emphysema. Group 4 consists of 15 patients (25%) with both airway obstruction and emphysema. Table 1 shows the characteristics of four groups.
Figure 1.
Four groups of patients disclosed by the presence or absence of airway obstruction and/or emphysema. Group 1 (n=26) with no airway obstruction (AO) and no emphysema are presented as hollow circles; group 2 (n=7) with emphysema and no AO, full circles; group 3 (n=11) with AO and no emphysema, hollow triangles; and group 4 (n=15) with both AO and emphysema, full triangles.
Table 1.
Anthropometric, lung function, and CT measurements of four groups
The data are presented as median (interquartile range) or number (%).
BMI: body mass index; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; FEF25-75%: forced expiratory flow at 25~75% of FVC; LAA%: computed tomography (CT) measurement of the percentage of low attenuation area less than -950 Hounsfield Units.
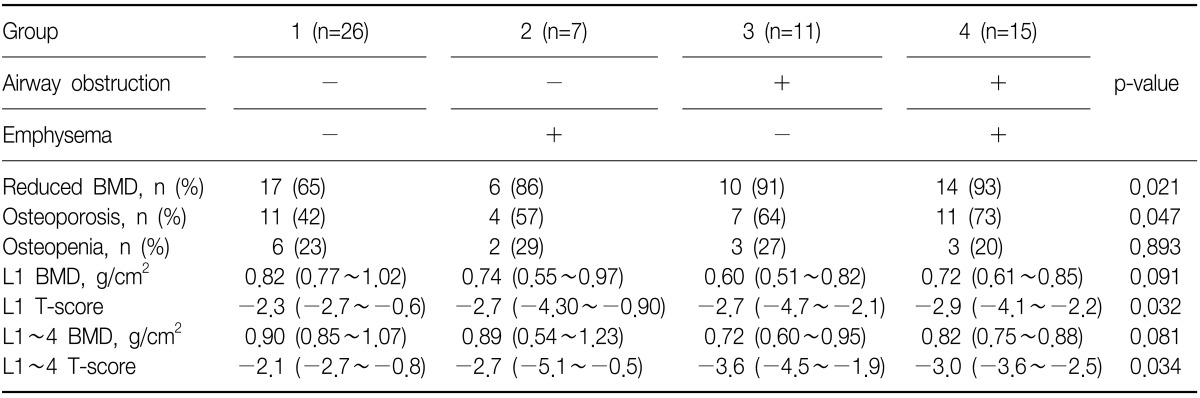
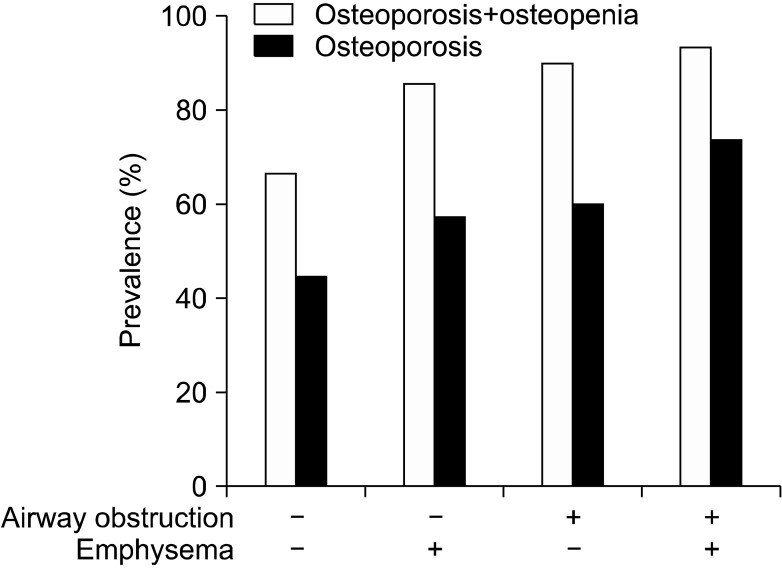
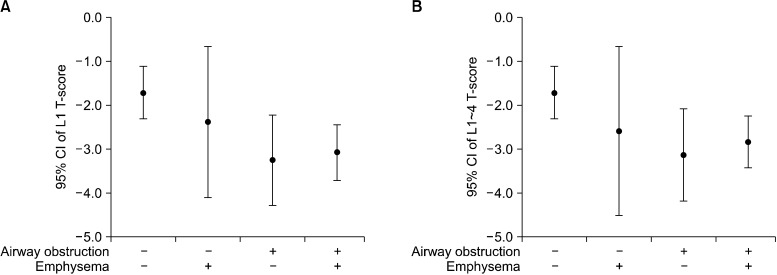
The prevalence of reduced BMD, including osteopenia and osteoporosis, and osteoporosis in group 1, 2, 3, and 4 were: 65%, 86%, 91%, and 93% (p=0.021 linear-by-linear association) and 42%, 57%, 64%, and 73% (p=0.047 linear-by-linear association), respectively (Table 2, Figure 2). T-scores of L1 (p=0.032) and L1~4 were different among four groups (p=0.034) (Table 2, Figure 3).
Table 2.
Prevalence of osteoporosis and parameters of BMD of four groups
The data are presented as median (interquartile range) or number (%).
BMD: bone mineral density; L: lumbar spine.
Figure 2.
Association between the presence of reduced bone mineral density (BMD) and four groups. The prevalence of reduced BMD, including osteopenia and osteoporosis, and osteoporosis in the four groups were: 65%, 86%, 91%, and 93% (p=0.021 linear-by-linear association) and 42%, 57%, 64%, and 73% (p=0.047 linear-by-linear association), respectively.
Figure 3.
(A, B) T-scores of L1 and L1~4 in four groups. CI: confidence interval.
BMI (p<0.001, p=0.001, p<0.001, and p<0.001) and FEF25-75%% predicted positively correlated with BMD of L1 and L1~4 and T-scores of L1 and L1~4, respectively (p=0.001, p=0.010, p=0.001, and p=0.007). A ratio of FEV1/FVC correlated with each of three BMD parameters except BMD of L1~4. FEV1% predicted correlated with BMD (p=0.030) and T-score of L1 (p=0.011). LAA%, the extent of emphysema, negatively correlated with only L1 T-score (p=0.035) (Table 3). However, by multiple linear regression analysis, body mass index (BMI) (p<0.001, p=0.001, p=0.001, and p=0.001) and FEF25-75% remained significantly associated with BMD of L1 and L1~4 and T-scores of L1 and L1~4 (p=0.012, p=0.026, p=0.019, and p=0.031).
Table 3.
Parameters of demography, lung function, and CT and correlation with BMD (n=59)
*Pearson's correlation coefficient.
BMD: bone mineral density; IQR: interquartile range; BMI: body mass index; FVC: forced vital capacity; FEV1: forced expiratory volume in one second; FEF25-75%: forced expiratory flow at 25~75% of FVC; LAA%: computed tomography (CT) measurement of the percentage of low attenuation area less than -950 Hounsfield Units.
Discussion
Cigarette smoking elicits airway inflammation in all of those who smoke, but in those who develop airflow obstruction there is persistent inflammation, even after smoking cessation16. This has led to the concept that an abnormal inflammatory response to cigarette smoke leads to the development of COPD in the susceptible individual. The spectrum of COPD is vast, with airway narrowing at one end and emphysema on the other, with the majority of patients existing somewhere in the middle. The reasons for the development of different phenotypes with the same exposure to cigarette smoke have not been elucidated. Possibly these phenotypes may exhibit a variety of spectrum or severity in extrapulmonary features. Even though not exactly, three of our four groups (Figure 1) could correspond to these phenotypes, emphysema, airway obstruction, and a mixture of these. Since emphysema patients without airway obstruction had markedly variable level of BMD shown in Figure 3 and the number of those was too small, we need further data. Patients with airway obstruction, even without emphysema, could have higher risk of reduced bone density than those with only emphysema. Similarly, while the prevalence of osteoporosis was the highest among subjects with both of airway obstruction and emphysema, it was higher among subjects with only airway obstruction than those with only emphysema. We assume that airway inflammation contributing to airway obstruction may closely related to systemic inflammation, which affects decrease in bone density.
In the present study, BMI is the most important factor associated with BMD. It is consistent with the results of previous studies1,2,4,6,7,9,10. In the general population, low BMI has also been identified as a risk factor for osteoporosis17. The link between low BMI and low BMD in COPD could be increased inflammation, decreased physical activity and/or other mechanisms leading to proteolysis18-20. If osteoporosis is a result of systemic inflammation of COPD, BMI rather than airway obstruction or emphysema is likely to reflect systemic inflammation and more important predictor of osteoporosis. Another explanation for more osteoporosis in patients with lower BMI could be that bone formation is decreased because there is relatively low mechanical loading on these bones. Indeed, astronauts lose as much bone mass in a 1-month spaceflight as postmenopausal females in 1 year21. In addition, COPD patients have been shown to be physically inactive compared with age-matched healthy subjects22.
Even though each of FEV1, FEV1/FVC, and the extent of emphysema was related to BMD, FEF25-75% showed significant association with BMD in our population. FEF25-75% is known to be a good indicator of small airway obstruction. It was reported to be lower in asymptomatic male smoker compared to male non-smokers and correlated with eosinophilic inflammation in childhood asthma23,24. Thus, it seems to be a marker of early airway inflammation induced by smoking or allergy. Our study design including subjects without airway obstruction may let FEF25-75% be more associated with BMD than FEV1. On the other hand, the extent of emphysema lost statistical significance when using multiple linear regression analysis. It is different from the study of Ohara et al.9. They showed that the extent of emphysema and BMI were predictive of BMD rather than FEV1. The discordance may be due to different study population. While our study included not only COPD patients but also subjects without COPD and female, they studied only male COPD patients. A lower FEV1 has been shown to be correlated with a low BMD7,10. Also, in subjects without COPD, significant correlations between FEV1 and BMD have been found25-27. These relationships between lung function parameters and BMD are complex and not yet clear. Again, in COPD patients, systemic inflammation can be a key factor, as reduced lung function has been found to be associated with increased inflammatory markers, which is a risk factor for osteoporosis28. However, emphysema is not correlated with functional impairment as much as spirometric parameters and expected to lesser reflect process of systemic inflammation. It is also possible that there is no causal relationship between lung function and BMD. Perhaps reduced physical activity because of impaired lung function is the reason for reduced BMD29.
There are several limitations in this study. The most important limitation was a small number of subjects. However, even with the relatively small population, we were able to demonstrate the strong correlations between the several parameters and BMD. Second, this study might have selection bias because of small sample size and controls being hospital user. However, results of this study are not different from previous study that BMD was related to more function of patients including disability of exercise due to dyspnea from airway obstruction than anatomical structure as emphysema. Third, we retrospectively selected subjects who had performed all three of BMD, spirometry, and CT. This might make selection bias and result in insufficient data collection of history of smoking, medication, and associated diseases. We did not have any data of osteoporosis-related biomarkers. Fourth, our classification not exactly corresponds to traditional phenotypes of COPD. However, in clinical practice, we often meet patients with emphysema and no airway obstruction. Fifth, we used FEV1/FVC rather than FEV1/VC recommended by American Thoracic Society/European Respiratory Society (ATS/ERS) guideline30. Even LLN for the FEV1/FVC ratio is enough to reduce the possibility of over-diagnosis of airway obstruction in our subjects over 40 years particularly compared to a fixed ratio of FEV1/FVC <0.712,13. Finally, postbronchodilator response was not measured. This study might include some subjects with reversible obstruction. Large randomized-controlled study should be required, later.
Our study suggested that BMI is the most important factor associated with reduced bone density in adults over 40 years regardless of airway obstruction and/or emphysema. Osteoporosis is more prevalent in patients with airway obstruction and emphysema. Airway obstruction seems to be more associated with bone density rather than does the extent of emphysema possibly due to physical inactivity and systemic inflammation.
Acknowledgements
This article was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A040153).
References
- 1.Iqbal F, Michaelson J, Thaler L, Rubin J, Roman J, Nanes MS. Declining bone mass in men with chronic pulmonary disease: contribution of glucocorticoid treatment, body mass index, and gonadal function. Chest. 1999;116:1616–1624. doi: 10.1378/chest.116.6.1616. [DOI] [PubMed] [Google Scholar]
- 2.Katsura H, Kida K. A comparison of bone mineral density in elderly female patients with COPD and bronchial asthma. Chest. 2002;122:1949–1955. doi: 10.1378/chest.122.6.1949. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 4.Bolton CE, Cannings-John R, Edwards PH, Ionescu AA, Evans WD, Pettit RJ, et al. What community measurements can be used to predict bone disease in patients with COPD? Respir Med. 2008;102:651–657. doi: 10.1016/j.rmed.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, et al. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183:885–890. doi: 10.1164/rccm.201004-0666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Incalzi RA, Caradonna P, Ranieri P, Basso S, Fuso L, Pagano F, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease. Respir Med. 2000;94:1079–1084. doi: 10.1053/rmed.2000.0916. [DOI] [PubMed] [Google Scholar]
- 7.Kjensli A, Mowinckel P, Ryg MS, Falch JA. Low bone mineral density is related to severity of chronic obstructive pulmonary disease. Bone. 2007;40:493–497. doi: 10.1016/j.bone.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Maggi S, Siviero P, Gonnelli S, Schiraldi C, Malavolta N, Nuti R, et al. Osteoporosis risk in patients with chronic obstructive pulmonary disease: the EOLO study. J Clin Densitom. 2009;12:345–352. doi: 10.1016/j.jocd.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Ohara T, Hirai T, Muro S, Haruna A, Terada K, Kinose D, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134:1244–1249. doi: 10.1378/chest.07-3054. [DOI] [PubMed] [Google Scholar]
- 10.Vrieze A, de Greef MH, Wijkstra PJ, Wempe JB. Low bone mineral density in COPD patients related to worse lung function, low weight and decreased fat-free mass. Osteoporos Int. 2007;18:1197–1202. doi: 10.1007/s00198-007-0355-7. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 12.Hwang YI, Kim CH, Kang HR, Shin T, Park SM, Jang SH, et al. Comparison of the prevalence of chronic obstructive pulmonary disease diagnosed by lower limit of normal and fixed ratio criteria. J Korean Med Sci. 2009;24:621–626. doi: 10.3346/jkms.2009.24.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 14.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 16.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26:835–835. doi: 10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd AJ, Cass AR, Carlson CA, Ray L. Development and internal validation of the male osteoporosis risk estimation score. Ann Fam Med. 2007;5:540–546. doi: 10.1370/afm.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramanian VP, Varkey B. Chronic obstructive pulmonary disease: effects beyond the lungs. Curr Opin Pulm Med. 2006;12:106–112. doi: 10.1097/01.mcp.0000208449.73101.ac. [DOI] [PubMed] [Google Scholar]
- 19.Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, Griffiths TL, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1414–1418. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 20.Wouters EF. Local and systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:26–33. doi: 10.1513/pats.200408-039MS. [DOI] [PubMed] [Google Scholar]
- 21.Cavanagh PR, Licata AA, Rice AJ. Exercise and pharmacological countermeasures for bone loss during long-duration space flight. Gravit Space Biol Bull. 2005;18:39–58. [PubMed] [Google Scholar]
- 22.Van Vliet M, Spruit MA, Verleden G, Kasran A, Van Herck E, Pitta F, et al. Hypogonadism, quadriceps weakness, and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:1105–1111. doi: 10.1164/rccm.200501-114OC. [DOI] [PubMed] [Google Scholar]
- 23.McKarns SC, Smith CJ, Morton MJ, Payne VM, Davis DL, Stringer LW, et al. Correlation of hematologic markers of inflammation and lung function: a comparison of asymptomatic smokers and nonsmokers. Hum Exp Toxicol. 1996;15:523–532. doi: 10.1177/096032719601500611. [DOI] [PubMed] [Google Scholar]
- 24.Rao R, Frederick JM, Enander I, Gregson RK, Warner JA, Warner JO. Airway function correlates with circulating eosinophil, but not mast cell, markers of inflammation in childhood asthma. Clin Exp Allergy. 1996;26:789–793. [PubMed] [Google Scholar]
- 25.Choi JW, Pai SH. Association between respiratory function and osteoporosis in pre- and postmenopausal women. Maturitas. 2004;48:253–258. doi: 10.1016/j.maturitas.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Lekamwasam S, Trivedi DP, Khaw KT. An association between respiratory function and bone mineral density in women from the general community: a cross sectional study. Osteoporos Int. 2002;13:710–715. doi: 10.1007/s001980200097. [DOI] [PubMed] [Google Scholar]
- 27.Lekamwasam S, Trivedi DP, Khaw KT. An association between respiratory function and hip bone mineral density in older men: a cross-sectional study. Osteoporos Int. 2005;16:204–207. doi: 10.1007/s00198-004-1673-7. [DOI] [PubMed] [Google Scholar]
- 28.Gan WQ, Man SF, Sin DD. The interactions between cigarette smoking and reduced lung function on systemic inflammation. Chest. 2005;127:558–564. doi: 10.1378/chest.127.2.558. [DOI] [PubMed] [Google Scholar]
- 29.Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008;177:743–751. doi: 10.1164/rccm.200707-1011OC. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]