FIG. 2.
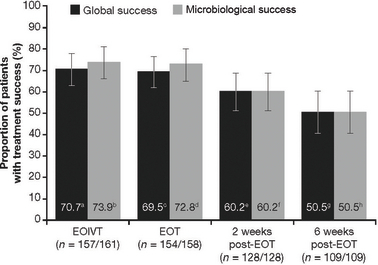
Global and microbiological success rates (with 95% confidence intervals) in modified intent-to-treat patients at the end of intravenous therapy (EOIVT), end of therapy (EOT), 2 weeks post EOT and 6 weeks post EOT. Missing and unknown global or microbiological responses were excluded in these analyses. a95% confidence interval (CI), 62.9–77.7. b95% CI, 66.4–80.5. c95% CI, 61.6–76.6. d95% CI, 65.1–79.6. e95% CI, 51.1–68.7. f95% CI, 51.1–68.7. g95% CI, 40.7–60.2. h95% CI, 40.7–60.2.
