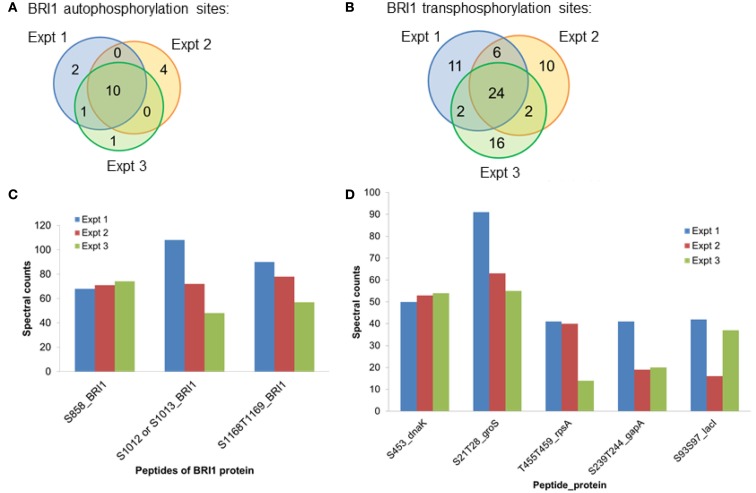
Figure 8.
The reproducibility of the proteomics analysis in this study. Three biological replicates of BRI1 auto- and transphosphorylated E. coli cells were digested with trypsin. Phosphopeptides were enriched with the TiO2 method, before identification with data-dependent analysis in the mass spectrometry. (A) Venn diagram showing the overlap of the identified BRI1 autophosphorylation peptides from three biological replicates. Ten abundant BRI1 autophosphorylation peptides were commonly identified. (B) Venn diagram showing the overlap of the identified BRI1 transphosphorylation peptides from three biological replicates. Twenty-four abundant BRI1 transphosphorylation peptides were commonly identified. (C) The reproducibility of spectral counts of three most abundant BRI1 autophosphorylation peptides using TiO2 enrichment. (D) The reproducibility of spectral counts on the five most abundant BRI1 transphosphorylation peptides using TiO2 enrichment. Note that phosphopeptides are identified using the single letter abbreviations for Ser (S) and Thr (T).