Abstract
Ganoderma fungus (Ganodermataceae) is a multifunctional medicinal mushroom and has been traditionally used for the treatment of various types of disease. Ganoderic acid DM (1) is a representative triterpenoid isolated from G. lingzhi and exhibits various biological activities. However, a universal starting point that triggers multiple signaling pathways and results in multifunctionality of 1 is unknown. Here we demonstrate the important clues regarding the mechanisms underlying multi-medicinal action of 1. We examined structure–activity relationships between 1 and its analogs and found that the carbonyl group at C-3 was essential for cytotoxicity. Subsequently, we used 1-conjugated magnetic beads as a probe and identified tubulin as a specific 1-binding protein. Furthermore, 1 showed a similar Kd to that of vinblastine and also affected assembly of tubulin polymers. This study revealed multiple biological activities of 1 and may contribute to the design and development of new tubulin-inhibiting agents.
“Lingzhi” in China and “reishi” in Japan, is a wood-rotting Ganoderma mushroom (Ganodermataceae) generally found growing on tree stumps. The anticancer activities of Ganoderma mushroom include inhibition of tumor growth, angiogenesis and metastasis, and immune enhancement1,2. Among these, the cytotoxic effects of Ganoderma triterpenoids and the immunoregulatory activities of Ganoderma polysaccharides have been of particular interest. Over one hundred oxygenated triterpenoids have been isolated from Ganoderma mushrooms3. These compounds display a wide range of biological activities resulting in prevention effect of diabetes mellitus4, cytotoxicity1 and antitumor activity2 and the inhibition of histamine release5 angiotensin converting enzyme release6, and cholesterol synthesis7.
It should be noted that molecular studies in recent years have revealed that the commercially cultivated ‘Ganoderma lucidum’ “Lingzhi” in East Asia is a different species from the true G. lucidum which originally described from Europe. Dai et al8 proposed a new species Ganoderma lingzhi Sheng H. Wu, Y. Cao & Y. C. Dai for “Lingzhi”, which has an East Asia distribution. Considering the characteristics of Ganoderma mushroom used in our study, we herein revised the scientific name of used mushroom from ‘G. lucidum’ to ‘G. lingzhi’ although we described ‘G. lucidum’ in our all previous papers9,10,11,12,13,14.
While screening mushrooms, we discovered that ethanol extracts of G. lingzhi showed the strongest 5α-reductase inhibitory activity among 19 species of mushrooms. Furthermore, treatment with the fruit body of G. lingzhi itself, or its ethanol extracts, significantly inhibited testosterone induced growth of the ventral prostate in rats9,10. Our group previously isolated a series of triterpenoids from G. lingzhi. These compounds suppressed the proliferation of androgen-dependent and androgen-independent prostate cancer cell lines11 and estrogen-dependent MCF-7 cells12, and inhibited osteoclastic differentiation13. Among these triterpenoids, we found that only ganoderic acid DM (1, Fig. 1) had multiple functions, such as 5α-reductase inhibition, androgen receptor binding activity, prostate cancer cell, and proliferation and osteoclast differentiation11,12,13,14. Although 1 affects different signaling pathways in different cell lines and has multiple functions, we have identified its target proteins, which explain and clarify the universal mechanism of its medicinal efficacy.
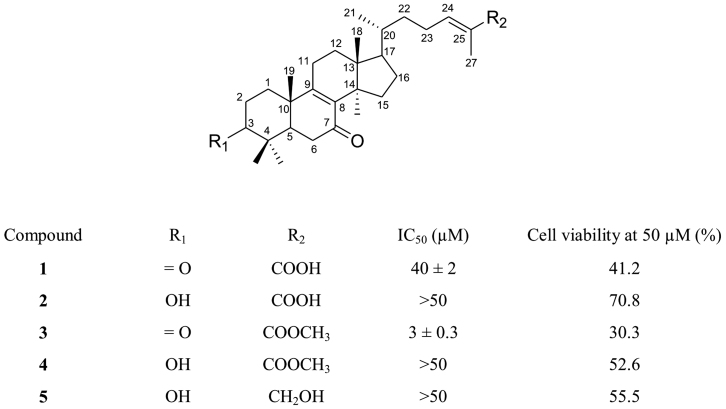
Figure 1. Chemical structures and antiproliferative activity of ganoderic acid DM (1) and its analogs (2–5).
Results
Initially, we investigated structure–activity relationships of 1 by synthesizing four analogs (Analogs 2–5; Fig. 1) and evaluated their antiproliferative activity in PC-3 cells. The results clearly indicate that substituents on C-3 and/or C-26 affect the antiproliferative activity of the parent compound 1 (Fig. 1). Among these five compounds, ganoderic acid DM methyl ester (3) showed the strongest antiproliferative activity. Compound 1 blocked 50% of cell proliferation at 40 μM, whereas 7-Oxo-ganoderic acid Z (2), 7-oxo-ganoderic acid Z methyl ester (4), and lucidadiol (5) all failed to inhibit cell proliferation to 50%, even at 50 μM. In contrast, the half inhibitory concentration (IC50) of compound 3 was only 3 μM. Hence, these compounds can be ranked in terms of their antiproliferative activity as follows: 3>1>4, 5 and 2. It can be concluded that the carbonyl group at C-3 is essential for eliciting the antiproliferative activity of ganoderic acid DM analogs.
Compounds 1 and 3 showed quite different solubility in cell culture medium. It should be noted that the poor aqueous solubility of 3 prevented investigation at concentrations greater than 50 μM. Thus, we became aware that the inhibitory activity of 3 on cell proliferation may be related to lipophilicity and cell membrane permeability. In general, the dissociated form of the carboxylic acid moeity on ganoderic acids improves aqueous solubility but hampers penetration of the plasma membrane lipid bilayer. At neutral pH conditions of cell medium, only a small amount of the dissociated form of 1 may enter into the cytoplasm of PC-3 cells to inhibit cell proliferation. If the specific part of the lanostane moiety in 1 is essential for inhibiting proliferation (not the carboxyl moiety at C-26), the change to the undissociated form of the carboxyl moiety, more specifically 3, should result in a more potent inhibitory activity than 1. Furthermore, structural modification of C-3 functional group of lanostane moiety of 1 decreases its inhibitory activity.
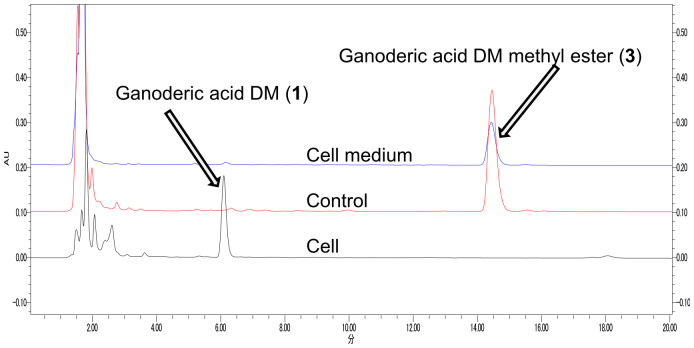
In subsequent experiments, PC-3 cells were treated with compounds 1 or 3 for 3 days, and the concentration of compounds 1 and 3 in cell culture medium and in cells was analyzed by HPLC. After treatment with 1, only compound 1 was detected in the cells. However, following treatment with 3, compound 1 was detected in cells, whereas 3 was only detected in the culture medium (Fig. 2).
Figure 2. HPLC analysis of ganoderic acid DM methyl ester (3) in cell extracts and culture medium.
Compound concentration: 20 μM;  : control (culture medium of wells without cells);
: control (culture medium of wells without cells);  : cell medium (culture medium with cells);
: cell medium (culture medium with cells);  : cells (cell extract).
: cells (cell extract).
This indicated that 3 effectively penetrates membranes and is metabolized completely to 1 by prostate cancer cells. On the other hand, when cells were treated with 1, only 1 could be found in the cell. These data confirm the superior membrane penetration of 3 and subsequent metabolism to 1, which elicits potent antiproliferative activity. Combined with the results of structure–activity analyses, it can be concluded that the carbonyl group at C-3 is essential for the inhibiting cell proliferation and that the methyl group at C-26 enhances penetration of cell membranes.
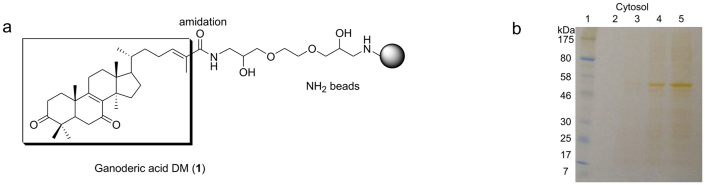
Recently, proteomic characterization of the cytotoxic mechanism of ganoderic acid D was investigated15. Furthermore, protein expression profiles in HeLa cells treated for 48 h with ganoderic acids F, K, B, D, and AM1 at 15 μM were examined using two-dimensional electrophoresis and MALDI-TOF MS/MS. This study identified target-related proteins, including human interleukin-17E, eukaryotic translation initiation factor 5A (eIF5A), peroxiredoxin 2, ubiquilin 2, Cu/Zn-superoxide dismutase, 14-3-3 α/β, TPM4-ALK fusion oncoproterin type 2, PP2A subunit A PR65 α isoform, nucleobinfin-1, heterogeneous nuclear ribonucleoprotein K, reticulocalbin 1, and chain A of DJ-1 protein16. Despite these data, direct molecular targets of triterpenoids remain elusive. Since 1 is effective for treatment of various cancer types, it must have a common molecular target and is involved in different signal pathways in different cancer types. To identify the primary target protein of 1, we used a technique involving ferrite glycidyl methacrylate (FG) beads to isolate the specific binding protein of 1. Keeping the essential C-3 carbonyl group free, compound 1 was covalently conjugated to the beads at C-26 and incubated with protein fractions of PC-3 cells (Fig. 3a). After extensive washing, the bound proteins were eluted and subjected to SDS gel electrophoresis and silver staining. No specific bands were detected from the membrane protein fraction (F2), nuclear protein fraction (F3), or cytoskeletal fraction (F4) (data not shown). Representative SDS gel images for control and 1-treated cytosol fractions (F1) showed a protein band at approximately 50 kD (Fig. 3b). This band was dependent on the treatment concentration of 1, and contained α,β-tubulin, as identified by LC-MS/MS. In these experiments, α-tubulin showed a total score of 1253, 61% sequence coverage and 45 matched peptides. Concomitantly, β-tubulin had a total score of 880, 45% sequence coverage and 27 matched peptides. Tubulin is a member of a small family of globular proteins, and the most abundant of these are α-tubulin and β-tubulin. Both of these proteins have a molecular weight of approximately 55 kD, and microtubules are assembled from dimers of α- and β-tubulin. Our results show that 1 specifically interacts with both α- and β-tubulin subunits, thereby effecting microtubule function.
Figure 3. SDS page image showing specific binding of cell protein to ganoderic acid DM (1) fixed with magnetic beads.
(A) Diagrams for ganoderic acid DM (1) fixation to the magnetic beads by reaction and amidation of the carboxylic group in the side chain of 1. (B) Lane 1: protein marker; lane 2: cytosolic protein incubated with FG beads; lane 3: cytosolic protein incubated with 1 (0.4 mM) bound FG beads; lane 4: cytosolic protein incubated with 1 (2 mM) bound FG beads; lane 5: cytosolic protein incubated with 1 (10 mM) bound FG beads. A specific binding protein of 46–58 kDa emerges with increased concentration of 1.
Unlike 1, compound 2 showed no affinity for tubulin protein (Table 1), thereby confirming again that the 3-carbonyl group is essential for antiproliferative activity. The dissociation constant of 1 was compared with that of vinblastine, and showed similar binding capacity at concentrations between 53 and 213 µM. In these experiments, we used vinblastine and paclitaxel as positive controls in a QCM system. This system used 50% EtOH as a solvent and to fix tubulin proteins in the sensor. It must be noted that these hydrophilic solvent conditions or the fixed point of tubulin may have undermined the affinity of paclitaxel for tubulin proteins.
Table 1. Tubulin Kd and reaction concentration of test compounds.
| Amount of Frequency shift/concentration (Hz x L/μmol) | |||||
|---|---|---|---|---|---|
| Vinblastine | 1 | 2 | Paclitaxel | ||
| Concentration (µM) | 53.3 | 0.2978 | 0.2901 | ||
| 106.5 | 0.19 | 0.1671 | |||
| 213 | 0.1415 | 0.148 | −0.0153 | 0.0259 | |
| Kd (µM) | 101 | 160 | |||
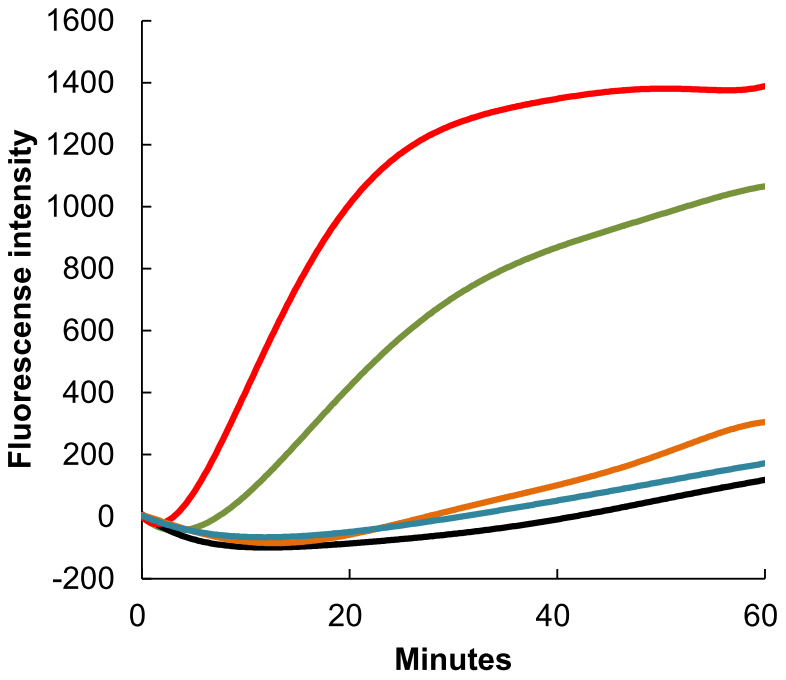
Cancer is characterized by uncontrolled cell proliferation and inappropriate cell survival, as well as defects in cellular morphogenesis that leads to tissue disruption, invasion, and migration. Microtubules play important roles in these cellular processes and comprise one of the oldest, most clearly validated, and efficacious targets for tumor chemotherapy. The formation of microtubules is a dynamic process that involves polymerization of heterodimers formed by α,β-tubulin, and degradation of linear polymers. Drugs that bind to tubulin can block this dynamic equilibrium, either by inhibiting polymerization or by stabilizing the microtubule structure. Both actions abolish microtubule function. To elucidate the mechanism through which 1 acts on tubulin protein, we developed a tubulin polymerization experiment. The effect of 1 on tubulin polymerization is shown in Fig. 4. We used paclitaxel and vinblastine as positive controls, as these agents stabilize the microtubule polymer and protect it from disassembly and suppress microtubule dynamics and reduce microtubule polymer mass, respectively. As we expected, paclitaxel or vinblastine caused increased assembly, or inhibited tubulin polymerization, respectively, at 30 µM. The concentration dependency of tubulin protein on 1 was reflected by increasing effects of 50–100 µM treatments of 1 on microtubule assembly. Unlike other tubulin-targeting drugs (vinblastine) that inhibit microtubule assembly, paclitaxel stabilizes the microtubule polymer and protects it from disassembly. This blocks progression of mitosis, prolongs activation of the mitotic checkpoint, and triggers apoptosis or reversion to the G-phase of the cell cycle without cell division. In support of our results, compound 1 was also shown to cause G1 cell cycle arrest and apoptosis in human breast cancer cells17. Tubulin-targeting by 1 triggers other signaling pathways in various cell types, explaining why this compound has multiple functions in prostate cancer cells and osteoclasts and induces benign prostate hyperplasia.
Figure 4. The effect of ganoderic acid DM (1) on tubulin polymer assembly:  : 1 at 100 μM;
: 1 at 100 μM;  : paclitaxel at 30 μM;
: paclitaxel at 30 μM;  : 1 at 50 μM;
: 1 at 50 μM;  : control;
: control;  : vinblastine at 30 μM.
: vinblastine at 30 μM.
Discussion
Cancer is a general term used to describe many disease states, each of which are characterized by abnormal cell proliferation. The causes of abnormal cellular behavior are specific to each type of cancer. Tubulin polymerizes into long chains or filaments that form microtubules, which are hollow fibers that serve as a skeletal system for living cells. Microtubules have the ability to shift through various formations, which enables a cell to undergo mitosis or to regulate intracellular transport. Binding to tubulin and causing the protein to lose its flexibility prevent a cell from dividing. These molecules inhibit cell mitosis by binding to tubulin in the mitotic spindle and preventing polymerization or depolymerization in microtubules. Thus, tubulin binding molecules have anticancer property and have generated significant interest in clinical oncology18,19.
In this study, we investigated structure–activity relationships of cytotoxic compound 1 and its analogs (2–5) in androgen-independent prostate cancer (PC-3) cells. Then we used affinity matrices, termed FG beads, to identify possible target proteins of 1. Tubulin was identified as a specific 1-binding protein using LC-MS/MS and showed a similar Kd to that of vinblastine; a famous tubulin-inhibiting agent. Compound 1 also increased the assembly of tubulin polymers. This study reveals that 1 targets tubulin to achieve multifunctional biological activities and anticancer activity. Furthermore, the results of our study seem to be consistent in part with those of previous proteomic studies of ganoderic acid D with the carbonyl group at C-3. They reported that microtubule-associated protein RP/EB family members such as cytokeratin 19, cytokeratin 1, and calumenin are possible ganoderic acid D target-related proteins. We speculate that binding to α, β-tubulin by 1 and other Ganoderma triterpenoids leads to a change in microtubule-related proteins and brings about abnormal cell proliferation. Though further research is required, the findings of this study provide important clues about the anticancer mechanism(s) of Ganoderma triterpenoids and should lead to the development of new chemotherapeutic agents based on the antitubulin activity of ganoderic acid DM and other Ganoderma triterpenoids.
Methods
Materials
Magnetic FG beads were obtained from Tamgawa Seiki Co., Kanagawa, Japan. Unless otherwise stated, all chemicals were purchased from Wako Co., Japan. The ProteoExtract subcellular proteome extraction kit was purchased from Merck Co., Japan. Vinblastin sulfate salt was obtained from Sigma-Aldrich, St. Louis, MO, USA. Tubulin (99% pure from porcine brain) was obtained from Cytoskeleton Inc., St. Denver, CO, USA. Ganoderic acid DM (1) is available from our previous works9,13,14.
Preparation of analogs (2–5) of ganoderic acid DM (1)
7-Oxo-ganoderic acid Z (2): Conversion of the C-3 carbonyl group of 1 to a C-3 hydroxyl group was performed by reduction with NaBH4. In brief, NaBH4 (10 mg) was added to 1 (10 mg) in methanol (3 ml) at room temperature. The mixture was stirred for 30 min at room temperature and the reaction was stopped by adding acetic acid (1 ml). The mixture was concentrated, and preparative HPLC (column, Inertsil ODS-3, 20 mm i.d. × 250 mm; methanol:water, 80:20; flow rate: 10 ml/min) afforded 2 (Rt, 10 min) as a white powder. The molecular formula of 2 was determined to be C30H46O4 on the basis of the ion peak at m/z 471.3508 [M+H+]+ in LCMS-IT-TOF spectra.
Ganoderic acid DM methyl ester (3): Compound 1 (10 mg) in methanol (1 ml)-benzene (3 ml) was added to trimethylsilyldiazomethane (1.5 ml) at room temperature. The mixture was stirred for 30 min at room temperature, concentrated, and preparative HPLC (column, Inertsil ODS-3, 20 mm i.d. × 250 mm; GL Science, Inc. USA; methanol:water, 80:20; flow rate: 10 ml/min) afforded 3 (Rt: 23 min). The molecular formula of 3 was determined to be C31H46O4 on the basis of the ion peak at m/z 483.3469 [M+H+]+ in LCMS-IT-TOF spectra.
7-Oxo-ganoderic acid Z methyl ester (4): Conversion of the C-3 carbonyl group of 3 to a C-3 hydroxyl group was performed by reduction with NaBH4. In brief, NaBH4 (10 mg) was added to 3 (10 mg) in methanol (3 ml) at room temperature. The mixture was stirred for 30 min at room temperature and the reaction was stopped by addition of acetic acid (1 ml). The mixture was concentrated, and preparative HPLC (column, Inertsil ODS-3; methanol:water, 80:20; flow rate: 10 ml/min) afforded 4 (Rt: 15 min) as a white powder. The molecular formula of 4 was determined to be C31H48O4 on the basis of the ion peak at m/z 485.3504 [M+H+]+ in LCMS-IT-TOF spectra.
Lucidadiol (5): Compound 5 was prepared from compound 2 by reduction with lithium aluminum hydride in anhydrous tetrahydrofurane (THF). In brief, a solution of 10 mg of 2 in dry THF (500 µl) was added slowly to a stirred dispersion of LiAlH4 (2.5 equivalents) at 0°C. After the addition, the reaction was allowed to reach room temperature and heated under reflux for 30 min until the starting material had disappeared, as monitored by HPLC. The reaction mixture was cooled to room temperature and then placed in an ice-water bath. Water was carefully added to remove excess LiAlH4, and finally 5N HCl solution was added. The crude mixture was extracted with ethyl acetate, dried over anhydrous Na2SO4, and the solvent was evaporated. The residue was purified by HPLC (column, Inertsil ODS-3; methanol:water, 80:20; flow rate: 10 ml/min) to afford 5 (Rt, 7 min) as a white powder. The molecular formula of 5 was determined to be C30H48O3 on the basis of the ion peak at m/z 459.0349 [M+H+]+ in LCMS-IT-TOF spectra.
Cell culture and cytotoxicity
Human prostate cancer (PC-3) cells were obtained from the American Type Culture Collection. The cells were used between passages 3 and 30, with a split ratio of 1:3 at each passage. The cells were plated into a 24-well plate with a 2 × 104/well density and supplemented with 5% steroid-depleted (DCC-stripped) cFBS. Twenty-four hours later, the cells were treated with either a vehicle control or various concentrations of test compounds. After 24 h, the culture medium was collected for HPLC analysis. New culture medium was added containing vehicle control or test compound. Seventy-two hours later, cell proliferation was determined by the 3-amino-7-dimethylamino-2-methyl-phenazine (NR) method. The NR solution was made at 5 mg/ml, and diluted with culture medium to 5 μg/ml. Culture medium was collected for HPLC analysis, NR solutions were added for 3 h at 37°C. NR solutions were then aspirated and the cells were washed with PBS twice. The NR extraction solution contained 50% ethanol and 1% acetic acid and was added (500 µl) to each well for 20 min at room temperature. The absorbance of each well was measured at 540 nm and cell extracts/NRsolutions were collected for HPLC analysis. The NR extraction solution was freeze dried, then extracted with 500 µl methanol. Methanol was evaporated and resolved in 50 µl of solvent (methanol:water, 80:20). These samples were analyzed by HPLC (column, Inertsil ODS-3; methanol:water, 80:20; flow rate, 1 ml/min; λ, 254 nm).
Preparation of 1-immobilized beads
Magnetic FG beads (NH2 beads; TASB848 Nii30; 5 mg) were incubated with 10 mM 1-hydroxybenzotriazole, 10 mM 1-ethyl-3-(3-demithyl-aminopropyl)-carbodiimide HCl, and various concentrations (0.4, 2, and 10 mM) of 1 in 1.5 ml of N,N-dimethylformamide for 4 h at room temperature. Unreacted residues were masked using 20% carbonic anhydride in N, N-dimethylformamide, and the resulting 1-immobilized beads (Fig. 3a) were stored at 4°C.
Cell fractionation
The ProteoExtract subcellular proteome extraction kit (S-PEK) was used in cell fractionation according to the manufacturer's instruction. S-PEK takes advantage of the different solubility of certain subcellular compartments in the four selected reagents. In this experiment, the procedure was performed directly in PC-3 culture dishes without the need for cell removal. Cells or the parts of the cells remain attached to the plate during sequential extraction of subcellular compartments until the appropriate extraction reagent is used. This procedure delivered four distinct protein fractions from PC-3. The four protein fractions were cytosolic fraction (F1), membrane protein fraction (F2), nuclear protein fraction (F3), and cytoskeletal fraction (F4).
Purification and identification of 1-binding protein
Compound 1-immobilized beads (the final concentrations of 1 were 0.4, 2, and 10 mM) and FG beads (without 1) were equilibrated with 0.5% NP-40 lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.5% NP-40. Cell protein fractions prepared from PC-3 were incubated with beads for 4 h at 4°C. Beads were washed five times with 100 mM KCl (200 µl). Then beads were washed with 1 M KCl (30 µl). This fraction was subjected to SDS-PAGE on a 10% gel. After performing silver staining of the gel (Wako, silver stain MS kit), the 50-kDa band in the cytosolic fraction was cut and treated with trypsin. Protein sequencing using mass spectrometry was performed as follows. Recovered peptides were analyzed using an electrospray ion trap mass spectrometer (LCQ, Finnigan MAT, San Jose, CA, USA) coupled on-line with nano-scale HPLC on a C18 column to acquire MS/MS spectra. A 0.1 × 50 mm-MAGICMS C18 column (5 μm particle diameter; 200 Å pore size) with mobile phases of A (methanol:water:acetic acid, 5:94:1) and B (methanol:water:acetic acid, 85:14:1) was used. Peptide mass fingerprinting was used for protein identification from tryptic fragment sizes using the Mascot search engine (http://www.matrixscience.com) querying to the entire NCBI database of theoretical human peptide masses.
QCM system
The affinity of compounds 1 and 2 for tubulin protein was tested using a QCM system. All compounds were dissolved in 50% EtOH. Paclitaxel and vinblastine sulfate salt (Sigma-Aldrich, St. Louis, MO, USA) were used as stabilizing and destabilizing controls, respectively. The response of these compounds against tubulin protein was investigated using a NAPiCOS QCM system (Nihon Dempa Kogyo Co. Ltd.) with a 30 MHz AT cut crystal twin sensor (Nihon Dempa Kogyo Co. Ltd.)20. As the sensor has two gold surface electrodes on one crystal, tubulin was physiologically fixed on one electrode (ch1) and the other one was absolutely blocked with BSA (ch2) for reference21. After stabilizing a baseline with PBS/50% EtOH, flow rate was set to 20 μl/min. One hundred microliters of each compound was injected into the system to react with fixed tubulin, and the differential frequency shift between ch1 and ch2 was obtained. Kd values were calculated by standard scatchard analysis with frequency changes from several concentrations of compounds22.
Tubulin polymerization assay
The effects of test compounds on tubulin polymerization were monitored using the standard assay protocol of a porcine tubulin based commercial kit (Tubulin Polymerization Assay Kit, Cytoskeleton Inc., Denver, CO, USA), which utilizes fluorescent reporter enhancement23. Fluorescence was measured using FlexStation3 (Molecular Devices, USA) and the test substance 1 (dissolved in DMSO) was evaluated at 50 and 100 µM. The experiment was performed twice (mean values are presented). Paclitaxel (Wako Co., Japan) and vinblastine were used as positive and negative controls, respectively.
Author Contributions
Jie Liu and Kuniyoshi Shimizu contributed equally to this work.
Acknowledgments
This study was supported by Kyushu University Interdisciplinary Programs in Education and Projects in Research Development (No.21306). The cost of publication was supported in part by the Research Grant for Young Investigators of Faculty of Agriculture, Kyushu University. We are grateful of Dr. H. Suhara and Mr. Kaneko for improving the manuscript.
References
- Lin C. N., Tome W. P. & Won S. J. Novel cytotoxic principles of Formosan Ganoderma lucidum. J. Nat. Prod. 54, 998–1002 (1991). [DOI] [PubMed] [Google Scholar]
- Iwatsuki K. et al. Lucidenic acids P and Q, methyl lucidenate P, and other triterpenoids from fungus Ganoderma lucidum and their inhibitory effects on Epstein-barr virus activation. J. Nat. Prod. 66, 1582–1585 (2003). [DOI] [PubMed] [Google Scholar]
- Shiao M. S. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem. Rec. 3, 172–180 (2003). [DOI] [PubMed] [Google Scholar]
- De Silva D. D., Rapior S., Hyde K. D. & Bahkali A. H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 56, 1–29 (2012). [Google Scholar]
- Kohda H. et al. The biologically active constituents of Ganoderma lucidum histamine release-inhibitory triterpenes. Chem. Pharm. Bull. 33, 1367–1374 (1985). [DOI] [PubMed] [Google Scholar]
- Morigiwa A., Kitabatake K., Fujimoto Y. & Ikekawa N. Angiotensin converting enzyme~inhibiting triterpenes from. Ganoderma lucidum. Chem. Pharm. Bull. 34, 3025–3028 (1986). [DOI] [PubMed] [Google Scholar]
- Komoda Y., Shimizu M., Sonoda Y. & Sato Y. Ganoderic acid and its derivatives as cholesterol synthesis inhibitors. Chem. Pharm. Bull. 37, 531–533 (1989). [DOI] [PubMed] [Google Scholar]
- Cao Y., Wu S. H. & Dai Y. C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 56, 49–62 (2012). [Google Scholar]
- Liu J., Kurashiki K., Shimizu K. & Kondo R. 5α-reductase inhibitory effects of triterpenoids isolated from Ganoderma lucidum. Biol. Pharm. Bull. 29, 392–395 (2005). [DOI] [PubMed] [Google Scholar]
- Fujita R. et al. Anti-androgenic activities of Ganoderma lucidum. J. Ethnopharmacol. 102, 107–112 (2005). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Anti-androgen effects of extracts and compounds from Ganoderma lucidum. Chem. Biodiversity. 6, 231–243 (2009). [DOI] [PubMed] [Google Scholar]
- Shimizu K. et al. Estrogen-like activity of ethanol extract of Ganoderma lucidum. J. Wood Sci. 55, 53–59 (2009). [Google Scholar]
- Liu J., Shiono J., Shimizu K. & Kondo R. Ganoderic acids from Ganoderma lucidum. Inhibitory activity of osteoclastic differentiation and structural criteria. Planta Med. 76, 137–139 (2010). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Ganoderic acid DM: anti-androgenic osteoclastogenesis inhibitor. Bioorg. Med. Chem. Lett. 19, 2154–2157 (2009). [DOI] [PubMed] [Google Scholar]
- Yue Q. X. et al. Proteomics characterization of the cytotoxicity mechanism of ganoderic acid D and computer-automated estimation of the possible drug target network. Mol. Cell Proteomics 7, 949–961 (2008). [DOI] [PubMed] [Google Scholar]
- Yue Q. X. et al. Effects of triterpenes from Ganoderma lucidum on protein expression profile of Hela cells. Phytomedicine 17, 606–613 (2010). [DOI] [PubMed] [Google Scholar]
- Wu G. S. et al. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 83(2), 408–414 (2012). [DOI] [PubMed] [Google Scholar]
- Jordan M. A. & Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4, 253–256 (2004). [DOI] [PubMed] [Google Scholar]
- Kingston D. G. I. Tubulin-interactive natural products as anticancer agents (1). J. Nat. Prod. 72(3), 507–515 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto T., Watanabe S., Wakamatsu S. & Koyama M. Examination for realization of a high precision crystal sensor. IEEE IFCS 2, 532–534 (2008). [Google Scholar]
- Mizutani N., Korposh S., Selyanchyn R., Wakamatsu S. & Lee S. W. Application of a quartz crystal microbalance (QCM) twin sensor for selective label-free immunoassay to simultaneous antigen-antibody reactions. Sensors & Transducers 137, 1–9 (2012). [Google Scholar]
- Ersöz A., Diltemiz S. E., Ozcan A. A., Denizli A. & Say R. Synergie between molecular imprinted polymer based on solid-phase extraction and quartz crystal microbalance technique for 8-OHdG sensing. Biosens Bioelectron 24, 742–747 (2008). [DOI] [PubMed] [Google Scholar]
- Bonne D., Heusele C., Simon C. & Pantaloni D. 4′,6-Diamidino-2-phenylindole, a fluorescent probe for tubulin and microtubules. J. Biol. Chem. 260, 2819–2825 (1985). [PubMed] [Google Scholar]