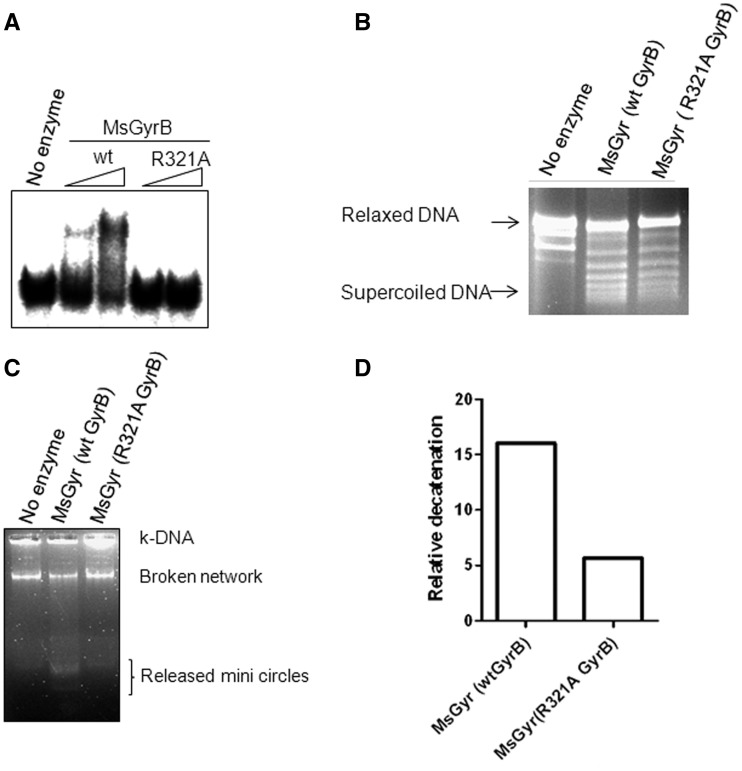
Figure 7.
DNA-binding activity of the wild-type (wt) and R321A GyrB subunit. (A) EMSA with 200 and 400 nM each of wt MsGyrB and its R321A mutant. The reaction conditions were similar to those used for Figure 2B. The 72-bp DNA was used as binding substrate; (B) DNA supercoiling of the mycobacterial gyrase reconstituted by mixing the wt or the R321A GyrB with GyrA subunit. An amount of 25 nM of M. smegmatis gyrase was reconstituted by mixing 25 nM of GyrA with 50 nM of either wt or R321A GyrB. The details of the reaction are given in ‘Materials and Methods’ section; (C) Decatenation assay with the holoenzyme reconstituted by mixing 25 nM of GyrA with 50 nM of either wt or R321A GyrB. An amount of 300 ng of kDNA was used as substrate. The decatenated minicircles were resolved on a 1.2% agarose gel; (D) Quantitation of the decatenation (released minicircles) by the wt and R321A GyrB containing holoenzyme from M. smegmatis (in Figure 7C) using Fujifilm multigauge V2.3.