Fig 3.
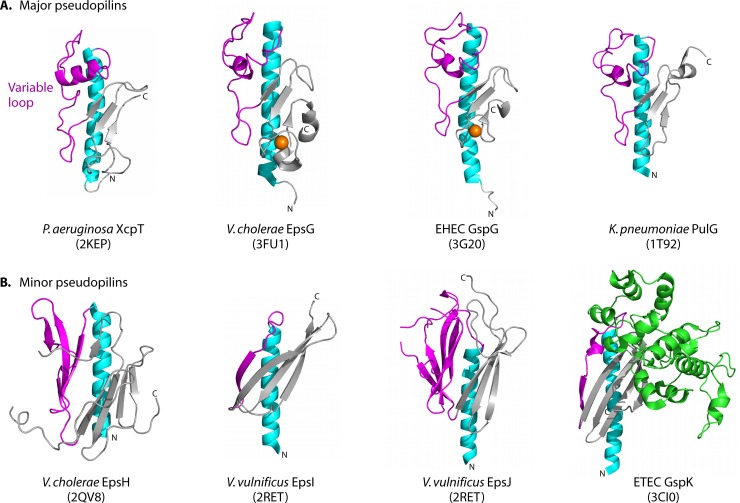
Structures of T2S pseudopilins and minor pseudopilins. T2S pseudopilins are structurally similar to the T4 pilins, with an N-terminal α-helix (cyan) connected to a β-sheet (gray) by a variable loop (magenta). (A) The major pseudopilins, represented by Pseudomonas aeruginosa XcpT (PDB accession no. 2KEP), Vibrio cholerae EpsG (PDB accession no. 3FU1), enterohemorrhagic E. coli GspG (PDB accession no. 3G20), and Klebsiella pneumoniae PulG (PDB accession no. 1T92), have variable loops with a helical character followed by a 3-stranded β-sheet. Near the C terminus is a calcium-binding motif (with a calcium ion shown in orange) in EpsG and GspG, although calcium binding is expected to occur in all major pseudopilins (235). (B) The minor pseudopilins, represented by GspH from V. cholerae (PDB accession no. 2QV8), GspI and GspJ from V. vulnificus (PBD accession no. 2RET), and GspK from enterotoxigenic E. coli (PDB accession no. 3CI0), vary in architecture, with a large α-domain insertion (green) in GspK. Figures were prepared using MacPymol (DeLano Scientific).