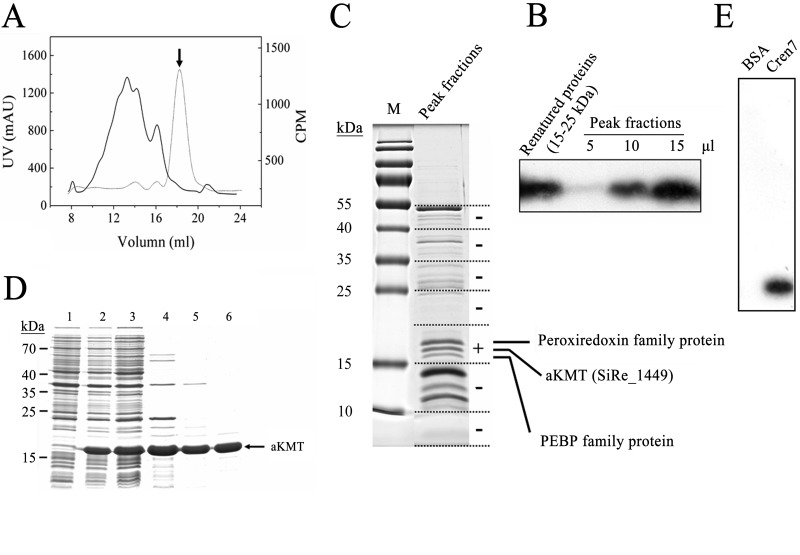
Fig 1.
Identification and purification of aKMT from Sulfolobus islandicus. (A) Gel filtration of a partially purified S. islandicus protein sample active in Cren7 methylation assays on Superdex G200. The sample had been through (NH4)2SO4 fractionation and successive chromatography on HiTrap Q and HiTrap S columns. The UV absorbance at 280 nm (solid line) and the methyltransferase activity (dotted line) of the column fractions are shown. The peak fraction, indicated by an arrow, displayed the highest methyltransferase activity. (B) Fluorographic analysis of methylation of Cren7 by the peak fractions and refolded proteins from the 15–25 kDa gel slice. Aliquots of the peak fractions and refolded samples from the 15-to-25-kDa gel slice were incubated with recombinant Cren7 in the presence of [3H]SAM. Proteins were precipitated with TCA, and the pellets were subjected to SDS-PAGE. The gel was processed for fluorography. (C) Separation of proteins in the peak fractions by SDS-PAGE and identification of proteins in a gel slice containing proteins active in Cren7 methylation assays by mass spectrometry. A sample of the peak fractions was resolved by 15% SDS-PAGE. The protein-containing portion of a sample lane was cut into slices, as shown by the dashed lines. Proteins in each gel slice were eluted, denatured, and renatured. The renatured samples were assayed for methyltransferase activity. The results of the assays are shown on the right. The gel slice that contained proteins active in the assay is indicated by a plus sign. The three visible protein bands in the slice showing methyltransferase activity were individually recovered from a separate gel, and each protein was identified by LC-MS/MS. (D) Overproduction and purification of recombinant aKMT protein. Samples taken at various steps during the overproduction and purification of aKMT were subjected to SDS-PAGE. Lane 1, total cellular proteins from cells before induction; lane 2, total cellular proteins from cells after induction; lane 3, supernatant of the cell lysate; lane 4, supernatant of the heat-treated sample; lane 5, flowthrough fractions of the HiTrap Q column; lane 6, fractions of the Superdex G75 column. Molecular mass markers are indicated on the left. (E) Methylation of Cren7 by recombinant aKMT. Recombinant aKMT was incubated with recombinant Cren7 in the presence of [3H]SAM. The samples were processed for fluorography as described above.