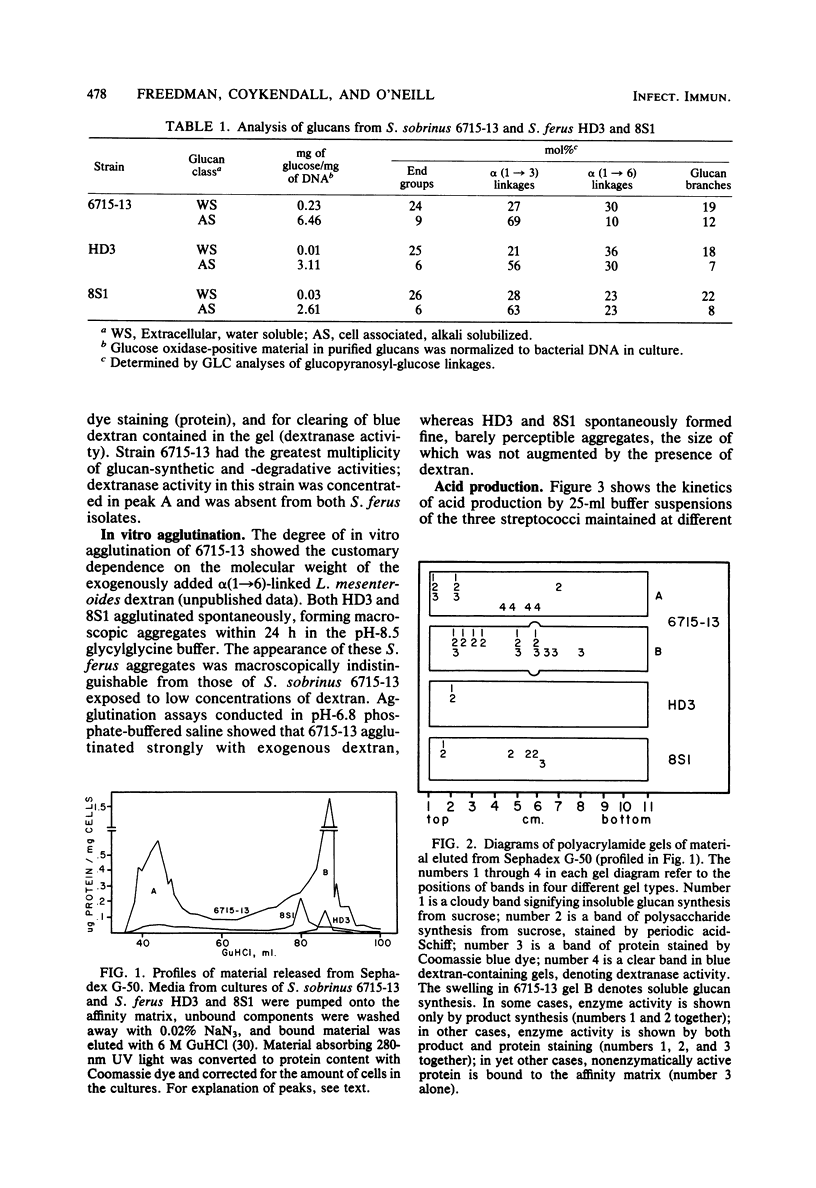
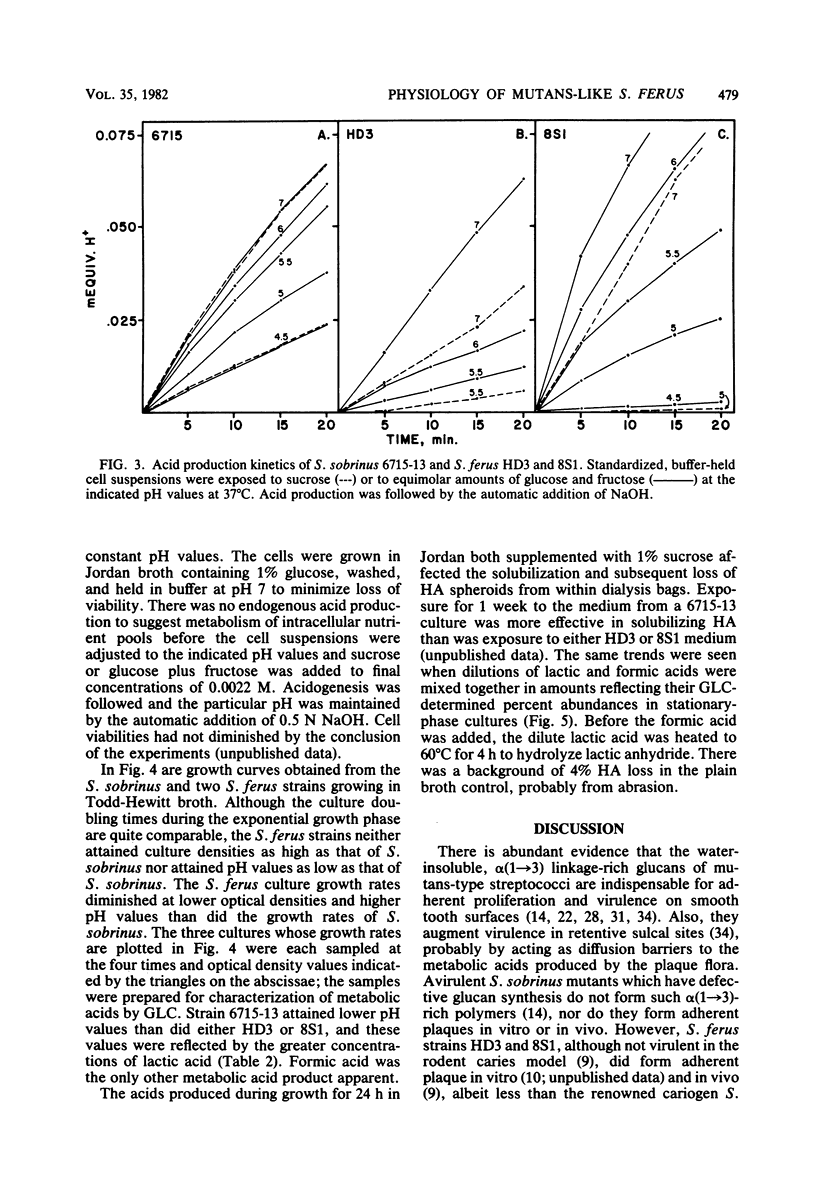
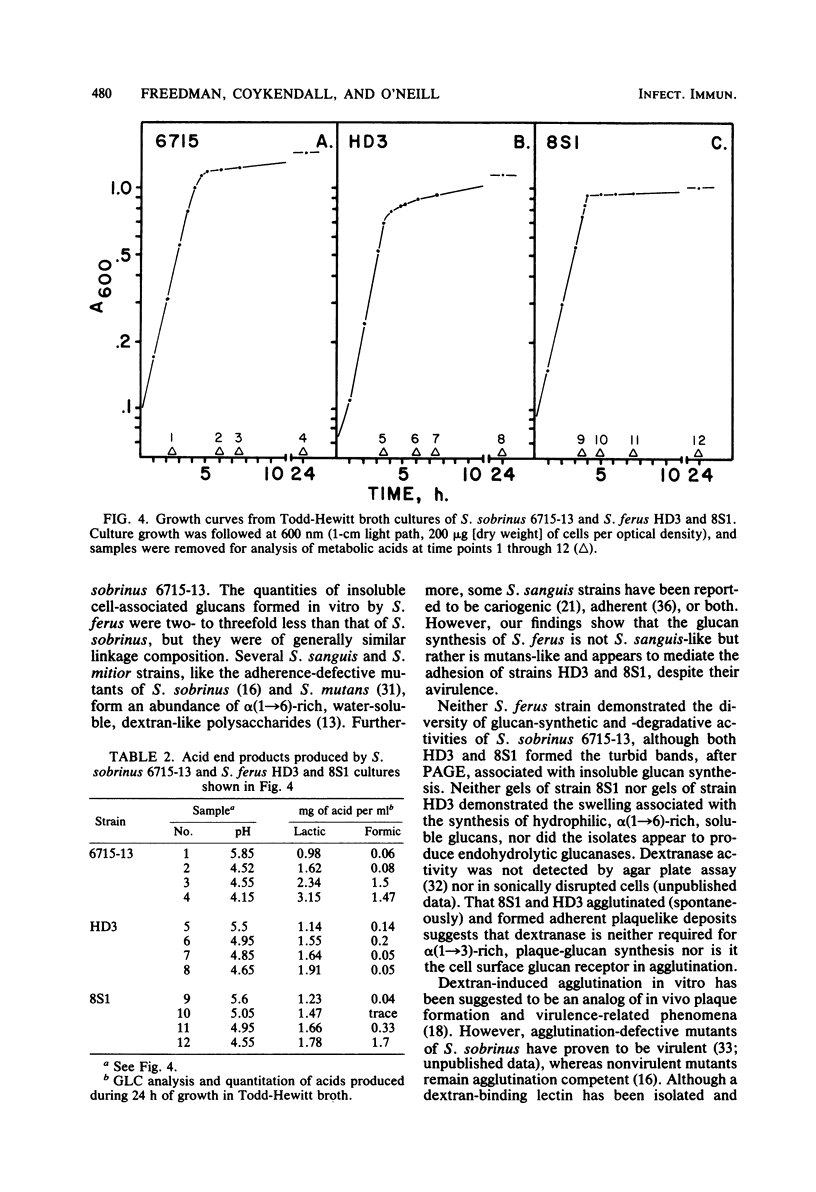
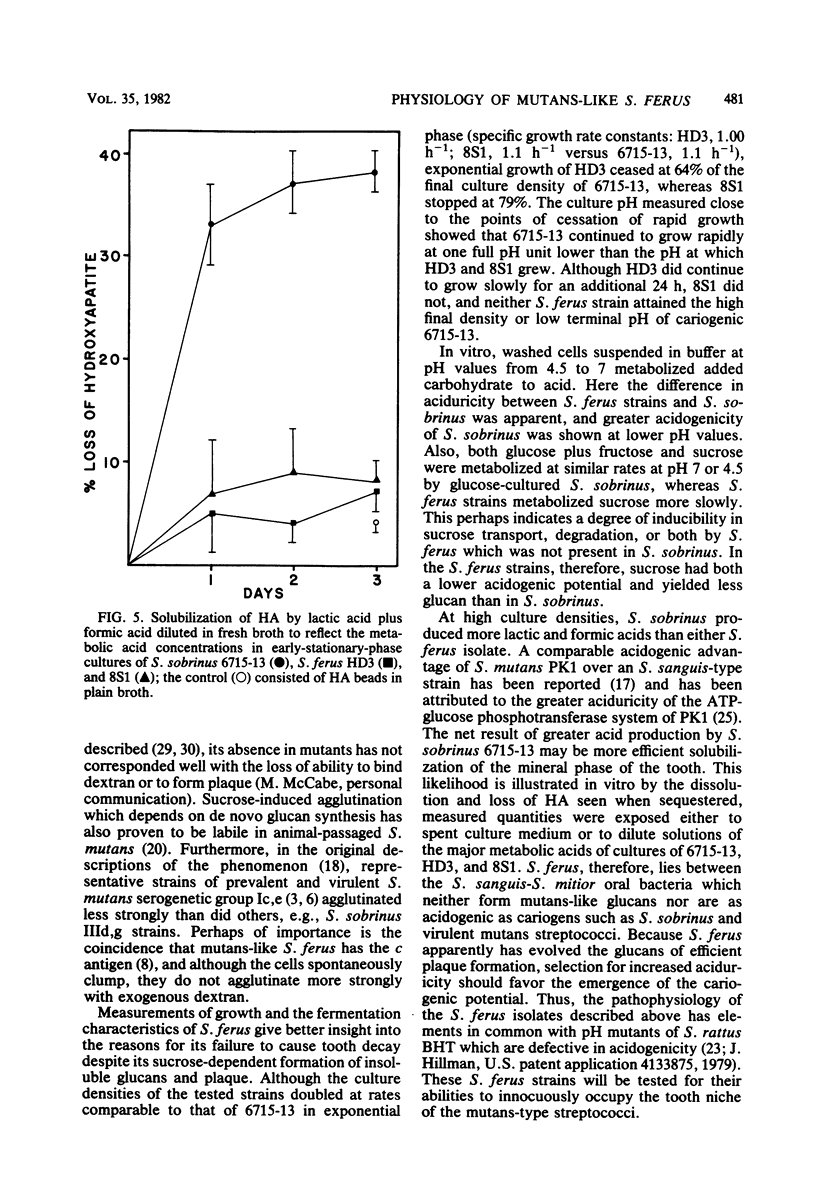
Abstract
Strains of Streptococcus ferus isolated from the oral cavities of wild rodents inhabiting sucrose-rich and sucrose-poor environments have many traits in common with the "mutans" streptococci. Thus, S. ferus HD3 and 8S1, like cariogenic S. sobrinus 6715-13, from adherent, alpha (1 leads to 3) glucopyranosyl-glucose linkage-rich, plaquelike deposits in vitro and in vivo through the action of constitutive glucosyltransferase(s) enzymes on sucrose, produce and degrade intracellular polysaccharide, produce short-chain fatty acids from the catabolism of mono- and disaccharides, carry the c antigen of S. mutans, and penetrate, persist, and proliferate in a sucrose-augmented fashion in the oral cavities of specific-pathogen-free rodent caries models. However, unlike infection with common S. mutans, infection with tested S. ferus strains does not cause caries. This avirulence appeared to result more from the reduced aciduricity of S. ferus than from differences in glucosyltransferase complements. Studies showed that despite generally similar growth rates and extracellular glucan syntheses, the acidogenic metabolism of S. ferus was more inhibited by declining environmental pH than was cariogenic S. sobrinus 6715-13 and that, in vitro, less hydroxyapatite was solubilized by S. ferus metabolic end products. The physiology of these S. ferus strains demonstrated that, in addition to plaque formation and acid production, acid tolerance was crucial to the carious process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Ciardi J. E., Hageage G. J., Jr, Wittenberger C. L. Multicomponent nature of the glucosyltransferase system of Streptococcus mutans. J Dent Res. 1976 Apr;55(Spec No):C87–C96. doi: 10.1177/002203457605500330011. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L., Bratthall D., O'Connor K., Dvarskas R. A. Serological and genetic examination of some nontypical Streptococcus mutans strains. Infect Immun. 1976 Sep;14(3):667–670. doi: 10.1128/iai.14.3.667-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Four types of Streptococcus mutans based on their genetic, antigenic and biochemical characteristics. J Gen Microbiol. 1974 Aug;83(2):327–338. doi: 10.1099/00221287-83-2-327. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L., Freedman M. L. Colonization and cariogenicity of Streptococcus ferus in rats. Infect Immun. 1981 Apr;32(1):80–85. doi: 10.1128/iai.32.1.80-85.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L., Specht P. A., Samol H. H. Streptococcus mutans in a wild, sucrose-eating rat population. Infect Immun. 1974 Jul;10(1):216–219. doi: 10.1128/iai.10.1.216-219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Coykendall A. L. Variation in internal polysaccharide synthesis among Streptococcus mutans strains. Infect Immun. 1975 Sep;12(3):475–479. doi: 10.1128/iai.12.3.475-479.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Birked D., Granath K. Analyses of glucans from cariogenic and mutant Streptococcus mutans. Infect Immun. 1978 Jul;21(1):17–27. doi: 10.1128/iai.21.1.17-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M., Birkhed D., Coykendall A., Rizzo D. Linkage analyses of extracellular glucans from Streptococcus sanguis and Streptococcus mitior. Infect Immun. 1979 Mar;23(3):907–909. doi: 10.1128/iai.23.3.907-909.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futakami K., Sato S., Iwami Y. Lactate formation at various ph levels by the wild strain of Streptococcus mutans Pk 1, its variant, and S sanguis. J Dent Res. 1976 Nov-Dec;55(6):1131–1131. doi: 10.1177/00220345760550062501. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Virulence-related physiological changes and antigenic variation in populations of Streptococcus mutans colonizing gnotobiotic rats. Infect Immun. 1980 Sep;29(3):1082–1091. doi: 10.1128/iai.29.3.1082-1091.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Hillman J. D. Lactate dehydrogenase mutants of Streptococcus mutans: isolation and preliminary characterization. Infect Immun. 1978 Jul;21(1):206–212. doi: 10.1128/iai.21.1.206-212.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami Y., Yamada T. Rate-limiting steps of the glycolytic pathway in the oral bacteria Streptococcus mutans and Streptococcus sanguis and the influence of acidic pH on the glucose metabolism. Arch Oral Biol. 1980;25(3):163–169. doi: 10.1016/0003-9969(80)90015-1. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- KEYES P. H., JORDAN H. V. PERIODONTAL LESIONS IN THE SYRIAN HAMSTER. III. FINDINGS RELATED TO AN INFECTIOUS AND TRANSMISSIBLE COMPONENT. Arch Oral Biol. 1964 Jul-Aug;9:377–400. doi: 10.1016/0003-9969(64)90024-x. [DOI] [PubMed] [Google Scholar]
- Koga T., Inoue M. Cellular adherence, glucosyltransferase adsorption, and glucan synthesis of Streptococcus mutans AHT mutants. Infect Immun. 1978 Feb;19(2):402–410. doi: 10.1128/iai.19.2.402-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M. Multiple forms of dextran-binding proteins from Streptococcus mutans. Adv Exp Med Biol. 1978;107:749–759. doi: 10.1007/978-1-4684-3369-2_84. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Shiota T., Ikeda T., Navia J. M., McGhee J. R. Virulence of Streptococcus mutans: biochemical and pathogenic characteristics of mutant isolates. Proc Soc Exp Biol Med. 1975 Nov;150(2):498–502. doi: 10.3181/00379727-150-39064. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Gawronski T. H., Schachtele C. F. Detection and preliminary studies on dextranase-producing microorganisms from human dental plaque. Infect Immun. 1973 Dec;8(6):1009–1016. doi: 10.1128/iai.8.6.1009-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L. Genetic alterations of Streptococcus mutans' virulence. Adv Exp Med Biol. 1978;107:661–672. doi: 10.1007/978-1-4684-3369-2_75. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren G., Freedman M. Comparative study of two variants of the mouth Streptococcus sanguis with different colonial morphologies and abilities to adhere. Arch Oral Biol. 1979;24(9):667–672. doi: 10.1016/0003-9969(79)90116-x. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]


