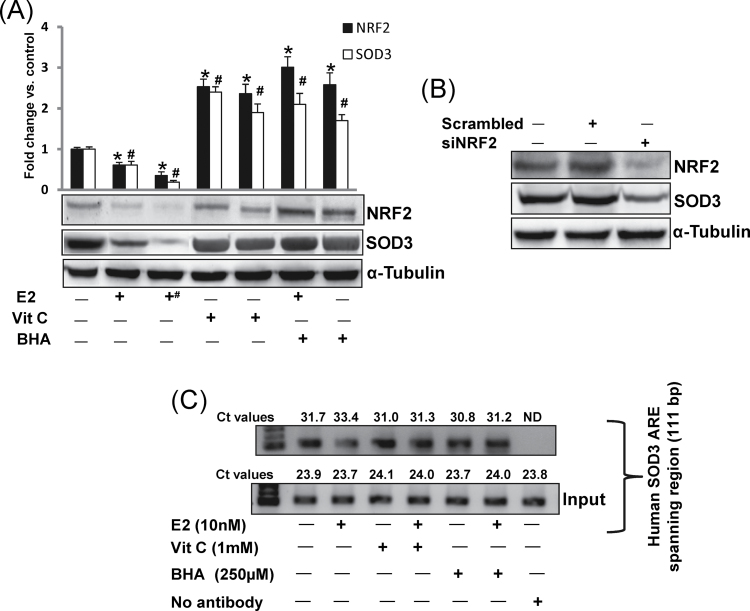
Fig. 3.
Antioxidants upregulate SOD3 via NRF2-dependent pathway. (A) Representative western blots are presented to show NRF2 and SOD3 protein expression in E2-exposed mammary tumors and in mammary tissues of rats treated with E2, Vit C or BHA for 240 days. The bar graph represents NRF2 and SOD3 protein fold change (mean ± SEM) in mammary tumors and in mammary tissues from at least three different animals compared with age-matched control mammary tissues. The symbol ‘+#’ indicates E2-induced mammary tumor tissues. The symbol ‘*’ indicates P value <0.05 for NRF2 protein expression compared with respective control mammary tissues, whereas the symbol ‘#’ indicates P value <0.05 for SOD3 protein expression compared with respective control mammary tissues. (B) MCF-10A cells were transfected with 20 nmol/l of scrambled siRNA or siNRF2 for 48h and western blot analyses were carried out using NRF2 antibody. Same membrane was reprobed with SOD3 and α-tubulin antibody. (C) MCF-10A cells were treated with Vit C (1mM) or BHA (250 μM) in the presence or absence of E2 (10nM), and vehicle for 3h, fixed with formaldehyde, cross-linked and chromatin sheared. The chromatin was immunoprecipitated with NRF2 antibody. NRF2 binding to ARE region of SOD3 promoter was analyzed by real-time PCR with specific primers for the human SOD3 ARE region. The ARE region of the SOD3 promoter was also amplified from 3 μl of purified soluble chromatin before immunoprecipitation to show input DNA. Representative ChIP agarose gels and real-time PCR Ct values from three independent experiments are shown (ND = not detected).