FIGURE 4.
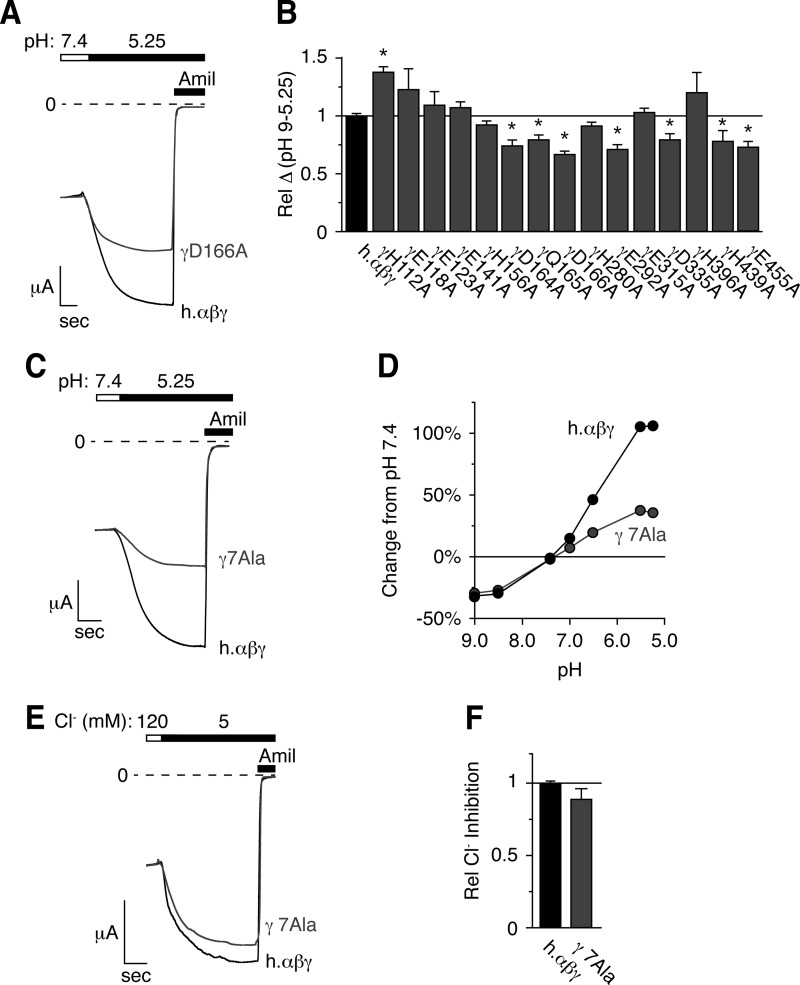
Mutation of titratable residues in the extracellular domain of γENaC decreases the response to acidic pH. A, C, and E, representative traces of current versus time recorded at −60 mV from Xenopus oocytes expressing human αβγENaC (A and C, black, scale bar = 1 μA, 5 s), human αβ- and γD166AENaC (A, gray, scale bar = 2 μA, 6.5 s), or human αβ- and γD164A,Q165A,D166A,E292A,D335A,H439A,E455AENaC (γ7Ala, C, gray, scale bar = 2 μA, 3.6 s). Currents were recorded under conditions of high Na+ (116 mm) and low extracellular Cl− (5 mm) over a range of pH from 9.0 to 5.25 (only pH 5.25 is shown). ENaC-dependent inward Na+ current was determined by application of amiloride at the end of the experiment. B, total pH response for each acidic residue identified in Fig. 3 (calculated as the difference in ENaC activity between pH 9.0 and 5.25) relative to wild type human αβγENaC (mean ± S.E. (error bars), n = 3–8, *, p < .05). D, dose-response relationship showing the change in ENaC activity versus extracellular pH relative to pH 7.4 for cells expressing wild type (black) and human αβγ7AlaENaC (gray, mean ± S.E., error bars are hidden by data symbols, n = 11). E, representative trace of Cl− inhibition recorded at −60 mV from oocytes expressing wild type (black) or αβγ7AlaENaC (gray). F, Cl− inhibition relative to wild type ENaC (mean ± S.E., n = 11).