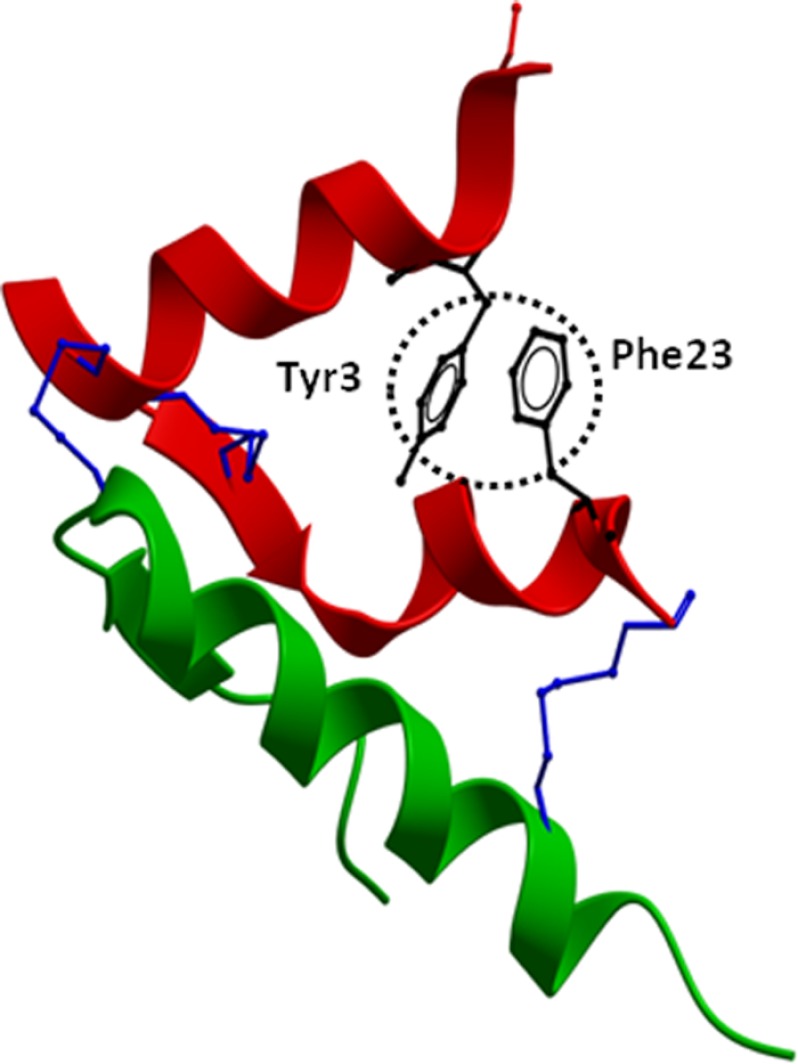
FIGURE 10.
Structure of native H2 relaxin. The crystal structure of H2 relaxin (Protein Data Bank code 6RLX) is shown with the side chains of Tyr-3 and Phe-23 (circled in black) that are present at opposite ends of the relaxin A-chain in close proximity. The A-chain is shown in red, the B-chain in green, and the conserved disulfide bridges in blue. The absence of Tyr-3 and/or Phe-23 contributes to the destabilization of the overall fold of the peptide. This causes loss of RXFP2 binding and activation because the peptide is unable to adopt an optimized orientation for binding to the receptor.