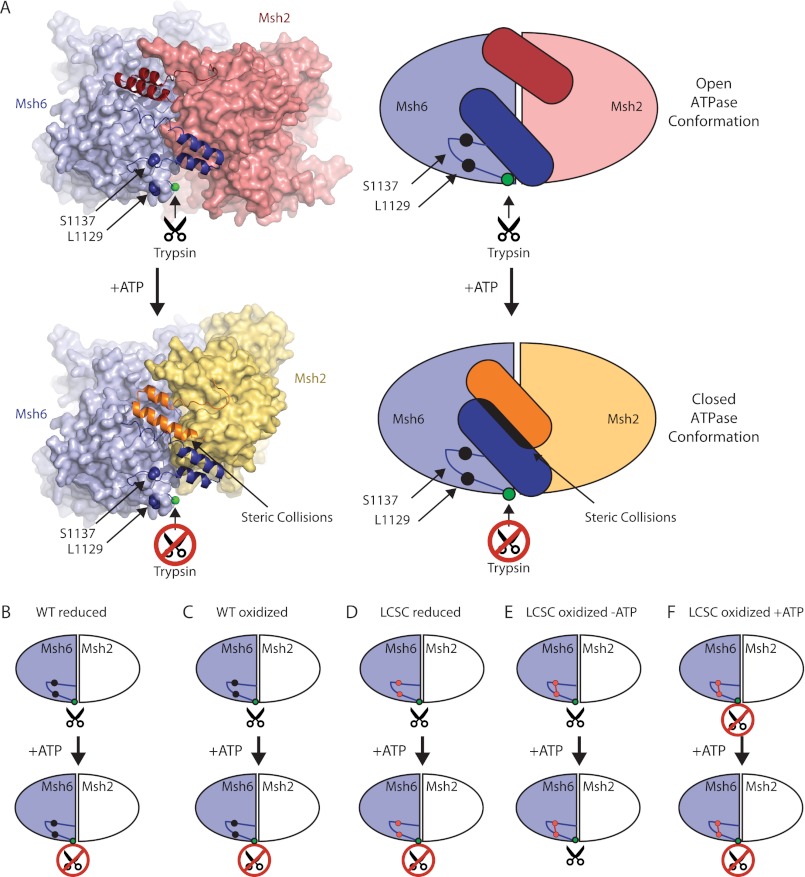
FIGURE 10.
Model of the open to closed transition of Msh2-Msh6 caused by ATP binding. A, diagram of human Msh2-Msh6 with the two C-terminal α helices in ribbons and the rest of the protein in spheres and a representative schematic. The ATP-bound conformation of human Msh2-Msh6 was modeled by individually superimposing the human Msh2 and Msh6 subunits (PDB code 2O8B) on the ATP-bound form of Pyrococcus furiosus Rad50 (PDB code 1F2U (43)) using Sequoia (60). Views were chosen so that the Msh6 orientation is fixed. Schematics of reduced wild-type Msh2-Msh6 (B), oxidized wild-type Msh2-Msh6 (C), reduced Msh2-Msh6 L1129C/S1137C (D), oxidized Msh2-Msh6 L1129C/S1137C (E), and ATP-supplemented oxidized Msh2-Msh6 L1129C/S1137C (F).