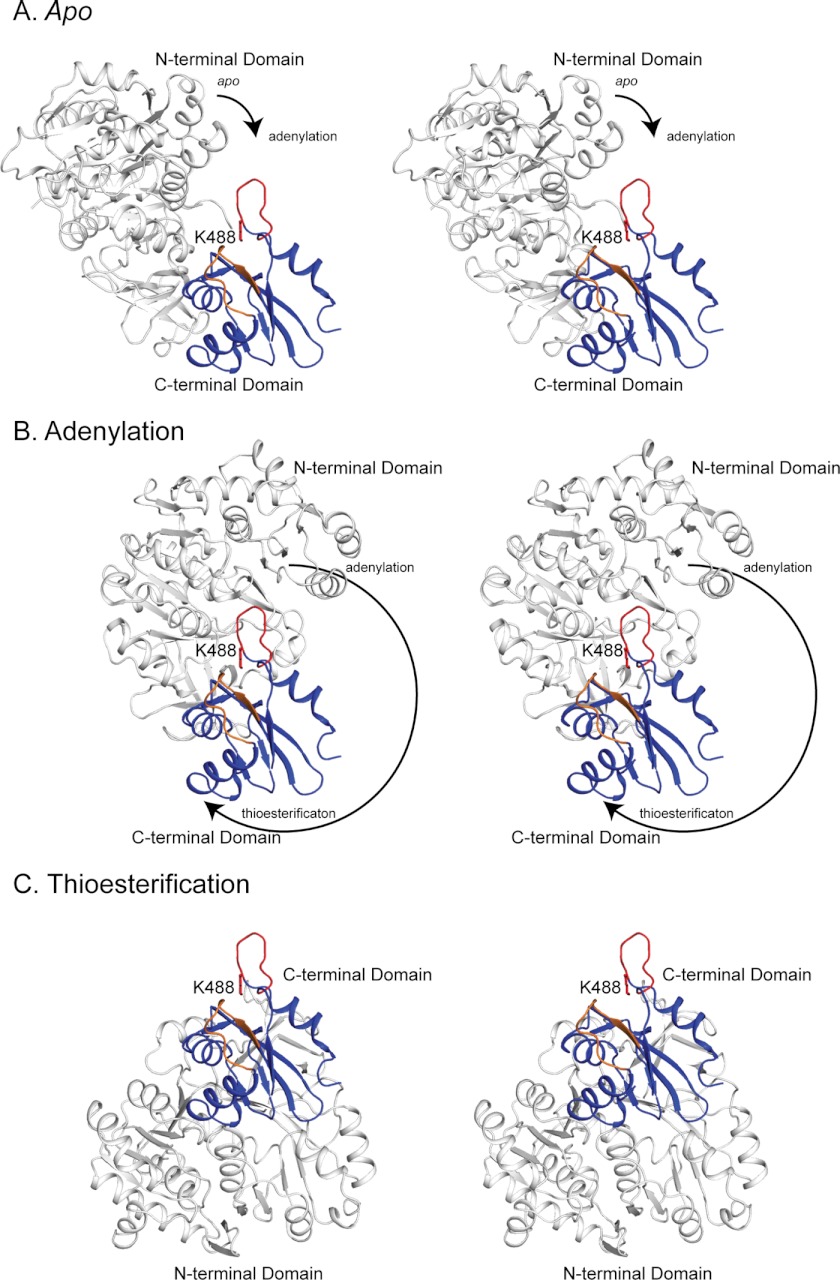
FIGURE 7.
Stereo view of the accessibility of Lys-488 in the apo (A), adenylation (B), and thioesterification (C) conformations. The N-terminal domain is shown in white and the C-terminal domain in blue. The active site loop and Lys-488 are shown in red, and the chimera loop is shown in orange. The Lys-488 side chain is modeled from the MatB apo structure. The C-terminal domains are shown in the same orientation in all three conformations. The arrows indicate the direction and magnitude of rotation of the N-terminal domain relative to the C-terminal domain during the transition from one conformation to the next. In all cases, the C-terminal domain is from the RpMatB-BxBclM B3 chimera structure, and the N-terminal domains are either from wild-type MatB (apo and thioesterification, PDB accession code 4FUQ) or MatBK488A (adenylation, PDB accession code 4FUT). In each case the N- and C-terminal domains are aligned to RpMatB structures (apo, 4FUQ; adenylation, 4FUT) or to the S. coelicolor MatB structure (thioesterification, 3NYQ (54)). Figure was prepared with PyMOL (60).