FIGURE 8.
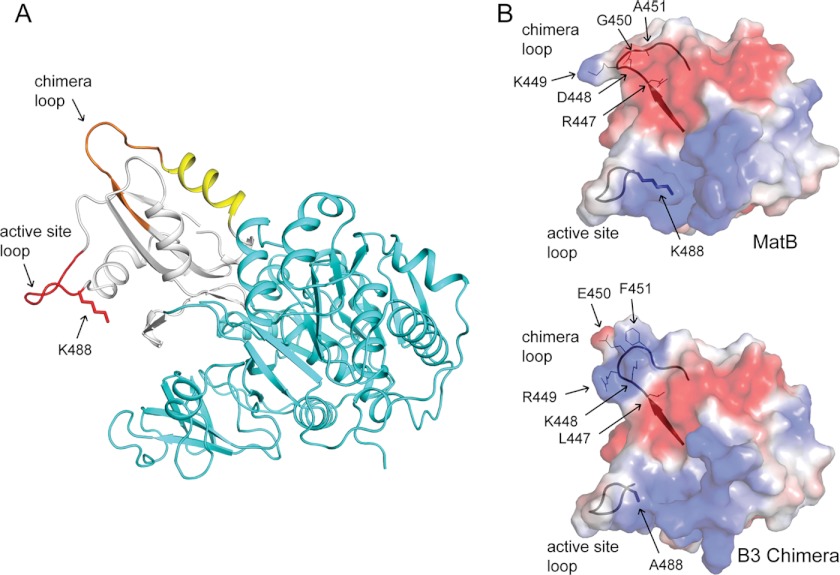
RpPat recognition elements of the RpMatB-BxBclM B3 chimera. A, RpMatB-BxBclM B3 chimera modeled in the thioester-forming conformation. The N-terminal domain is shown in cyan, the C-terminal domain in white, the active site loop in red, the residues derived from BxBclM in orange, and additional residues changed in the RpMatB-BxBclM B1 chimera are shown in yellow. The acetylation site, Lys-488, was modeled from the RpMatB apo structure. B, surface electrostatic calculations for the RpMatB and RpMatB-BxBclM B3 chimera C-terminal domains were generated using PyMOL (60), with electropositive regions shown in blue and electronegative regions shown in red. Ribbon diagrams of the active site and chimera loop backbones are shown for reference, and residues within the chimera loop are labeled. The active site lysine residue (Lys-488) was mutated to an alanine in the RpMatB-BxBclM B3 chimera structure to aid crystallization. Figure was prepared with PyMOL (60).