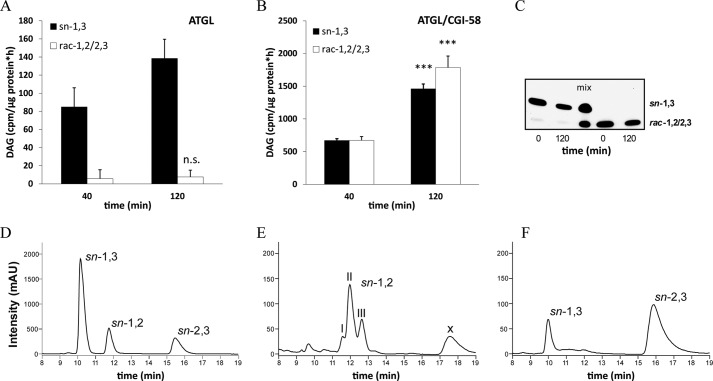
FIGURE 4.
ATGL specifically cleaves the sn-2 ester bond and expands its selectivity to the sn-1 position upon co-activation by CGI-58. A and B, cytosolic fractions of Cos-7 cells overexpressing ATGL were incubated in the absence (A) or presence (B) of GST-tagged CGI-58 with 3H-labeled triolein substrate in the presence of an HSL-specific inhibitor (76-0079) at 37 °C. Reactions were stopped at different time points, DAG regioisomers were separated by TLC, and radioactivity in the corresponding bands was measured by liquid scintillation counting. n.s., not significant. C, to assess transesterification of DAG isomers, sn-1,3 and rac-1,2/2,3 DAG were incubated with lysates of Cos-7 cells in the presence of an HSL-specific inhibitor (76-0079) for 120 min at 37 °C. DAG species were analyzed by TLC before and after incubation. D--F, chiral-phase HPLC resolution of different DAG species is shown. DAGs were analyzed as their corresponding 3,5-dinitrophenylurethanes by chiral-phase HPLC. Analysis of a racemic diolein reference mix is shown in D. Analysis of the reaction products of “egg yolk lecithin” hydrolyzed by purified PLC (B. cereus) is shown. The three peaks within the retention time range 11.5–13 min display the different FA composition of the separated DAGs; I, 16:0–18:1 + 18:1–18:1; II, 16:0–18:2 + 18:1–18:2; III, 18:2–18:2 (E). Analysis of the reaction products of triolein substrate hydrolyzed by CGI-58 co-activated ATGL contained in the cytosolic fraction of Cos-7 cells and in the presence HSL-specific inhibitor (76-0079, F). Data are normalized to LacZ and are presented as the means ± S.D. and are representative of two independent experiments (***, p < 0.001). X = unknown compound. mAU, milliabsorbance units.